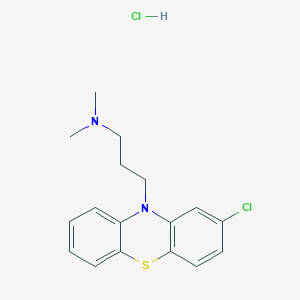
Chlorpromazine hydrochloride
Vue d'ensemble
Description
Le chlorhydrate de chlorpromazine est un puissant tranquillisant synthétique qui agit sélectivement sur les centres supérieurs du cerveau comme un dépresseur du système nerveux central. Il est principalement utilisé pour traiter les troubles psychotiques tels que la schizophrénie, le trouble bipolaire, les problèmes de comportement graves chez les enfants, les nausées et les vomissements, l'anxiété avant une intervention chirurgicale et les hoquets persistants .
Applications De Recherche Scientifique
Medical Uses
Chlorpromazine hydrochloride is indicated for several medical conditions, including:
- Psychotic Disorders : Primarily used for the treatment of schizophrenia, chlorpromazine helps alleviate both positive symptoms (hallucinations and delusions) and negative symptoms (apathy and lack of emotion) .
- Bipolar Disorder : Effective in managing acute manic episodes, chlorpromazine stabilizes mood and reduces excessive energy levels .
- Acute Agitation : It is utilized to control severe agitation and aggressive behavior, particularly in emergency settings .
- Nausea and Vomiting : Chlorpromazine is effective against nausea associated with surgery, chemotherapy, and terminal illnesses .
- Persistent Hiccups : The drug is FDA-approved for treating persistent singultus (chronic hiccups) lasting more than 48 hours .
- Adjunct Treatment : It serves as an adjunct in the treatment of tetanus and acute intermittent porphyria .
Table 1: Summary of Medical Indications for this compound
| Condition | Description |
|---|---|
| Schizophrenia | Management of both positive and negative symptoms |
| Bipolar Disorder | Control of acute manic episodes |
| Acute Agitation | Control of severe agitation and aggressive behavior |
| Nausea and Vomiting | Treatment for nausea related to surgery or chemotherapy |
| Persistent Hiccups | Treatment for hiccups lasting over 48 hours |
| Adjunct Treatment | Used in tetanus and acute intermittent porphyria |
Antiparasitic Effects
Recent studies have explored the repurposing of this compound for treating neglected tropical diseases such as Chagas disease. Research indicates that chlorpromazine exhibits growth inhibitory activity against Trypanosoma cruzi, the causative agent of Chagas disease. In vitro studies have shown that chlorpromazine can be combined with other FDA-approved drugs to enhance therapeutic efficacy against this parasite. For instance, combinations with other compounds have demonstrated synergistic effects, improving treatment outcomes in murine models .
Antifungal Activity
Chlorpromazine has also been investigated for its antifungal properties. Studies have demonstrated that it possesses in vitro antifungal activity against various fungal strains. This finding suggests potential applications in treating fungal infections, particularly in immunocompromised patients who are at higher risk for such infections .
Case Study 1: Schizophrenia Management
A systematic review involving multiple trials assessed the effectiveness of chlorpromazine compared to placebo in treating schizophrenia. Results indicated that patients receiving chlorpromazine experienced significantly fewer relapses over a follow-up period of up to two years. However, the review also highlighted the severe side effects associated with long-term use, necessitating careful monitoring and management .
Case Study 2: Chagas Disease Treatment
In a study aimed at repurposing existing medications for Chagas disease, researchers found that chlorpromazine combined with other drugs showed enhanced efficacy in reducing parasitemia in infected mice. This study emphasizes the potential of using established medications like chlorpromazine to develop new therapeutic strategies for diseases lacking effective treatments .
Mécanisme D'action
Target of Action
Chlorpromazine hydrochloride, a phenothiazine antipsychotic drug, primarily targets dopamine receptors in the brain . It is thought that the antipsychotic actions of chlorpromazine are due to long-term adaptation by the brain to blocking these receptors .
Mode of Action
Chlorpromazine acts as an antagonist on different postsynaptic receptors, primarily on dopaminergic-receptors (subtypes D1, D2, D3, and D4) . By blocking these receptors, chlorpromazine prevents dopamine from binding to its receptors, thereby reducing dopaminergic transmission within the brain . This action is believed to contribute to its antipsychotic effects .
Biochemical Pathways
The primary biochemical pathway affected by chlorpromazine is the dopaminergic pathway . By blocking dopamine receptors, chlorpromazine disrupts the normal functioning of this pathway, leading to reduced dopaminergic transmission . Additionally, it has been found that chlorpromazine can be metabolized by the cytochrome P450 isoenzyme 1A2, with N14-demethylation being the most thermodynamically and kinetically favorable metabolic pathway .
Result of Action
The molecular and cellular effects of chlorpromazine’s action primarily involve the reduction of dopaminergic transmission in the brain . This can lead to a decrease in the positive symptoms of psychosis, such as hallucinations and delusions . Additionally, chlorpromazine has been found to demonstrate cytotoxic and antiproliferative activity against leukemic cells .
Action Environment
Environmental factors can influence the action, efficacy, and stability of this compound. For instance, global warming and environmental pollution have been suggested to create a unique combination of abiotic and biotic stresses to non-target aquatic organisms in light of global warming . .
Analyse Biochimique
Biochemical Properties
Chlorpromazine hydrochloride plays a crucial role in biochemical reactions by acting as an antagonist on various postsynaptic receptors. It primarily interacts with dopaminergic receptors (subtypes D1, D2, D3, and D4), serotonergic receptors (5-HT1 and 5-HT2), histaminergic receptors (H1 and H2), and muscarinic receptors (M1 and M2) . These interactions result in the modulation of neurotransmitter activity, leading to its antipsychotic, antiemetic, and sedative effects.
Cellular Effects
This compound exerts significant effects on various types of cells and cellular processes. It influences cell function by modulating cell signaling pathways, gene expression, and cellular metabolism. For instance, this compound can initiate cell death in both cancerous and healthy liver cells by inducing apoptosis . Additionally, it affects neurogenesis in the rat brain by decreasing the number of Sox-2-expressing stem cells and early neural progenitors .
Molecular Mechanism
The molecular mechanism of this compound involves its action as an antagonist on different postsynaptic receptors. It blocks dopaminergic receptors, leading to a reduction in dopamine activity, which is associated with its antipsychotic properties . This compound also inhibits serotonergic, histaminergic, and muscarinic receptors, contributing to its anxiolytic, antidepressive, and antiaggressive effects . These interactions result in changes in gene expression and enzyme activity, further influencing cellular functions.
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of this compound change over time. The compound exhibits multicompartmental pharmacokinetics, with a half-life of approximately 11.05 hours . It undergoes extensive first-pass metabolism, leading to significant variability in its pharmacokinetics. Over time, this compound can degrade, affecting its stability and long-term impact on cellular functions .
Dosage Effects in Animal Models
The effects of this compound vary with different dosages in animal models. At therapeutic doses, it can cause side effects such as orthostatic hypotension, jaundice, and leukopenia . High doses may lead to toxic effects, including interference with pituitary-gonadal function and neurogenic stem/progenitor cell formation . These dosage-dependent effects highlight the importance of careful dosage management in clinical settings.
Metabolic Pathways
This compound is involved in several metabolic pathways, primarily catalyzed by cytochrome P450 isoenzymes. The main metabolic mechanisms include N-demethylation, S-oxidation, and aromatic hydroxylation . These pathways result in the formation of various metabolites, such as mono-N-desmethylchlorpromazine, chlorpromazine sulfoxide, and 7-hydroxychlorpromazine . These metabolites can further undergo phase II metabolism, including glucuronidation and sulfation .
Transport and Distribution
This compound is transported and distributed within cells and tissues through various mechanisms. It exhibits wide distribution in the body, with a volume of distribution at steady state of approximately 642 liters . The compound is highly protein-bound, with 90-99% of it bound to plasma proteins . This extensive binding affects its localization and accumulation in different tissues.
Subcellular Localization
The subcellular localization of this compound is influenced by its interactions with various biomolecules. It can localize to different cellular compartments, including the cytoplasm and cell membrane. The compound’s activity and function are affected by its localization, as it can interact with specific receptors and enzymes within these compartments . Post-translational modifications, such as phosphorylation, may also play a role in directing this compound to specific organelles.
Méthodes De Préparation
Voies de synthèse et conditions de réaction
Le chlorhydrate de chlorpromazine est synthétisé par un processus en plusieurs étapes. La synthèse commence par la réaction de la 2-chlorophénothiazine avec le chlorure de 3-diméthylaminopropyle en présence d'une base telle que l'amidure de sodium. Cette réaction forme la chlorpromazine, qui est ensuite convertie en son sel de chlorhydrate par traitement avec de l'acide chlorhydrique .
Méthodes de production industrielle
En milieu industriel, le chlorhydrate de chlorpromazine est produit selon des voies de synthèse similaires, mais à plus grande échelle. Le processus consiste à mélanger la chlorpromazine avec de la cellulose microcristalline, du monohydrate de lactose, de l'amidon prégélatinisé, de la silice colloïdale et du stéarate de magnésium dans des conditions spécifiques. Le mélange est ensuite tamisé et comprimé pour obtenir la formulation pharmaceutique finale .
Analyse Des Réactions Chimiques
Types de réactions
Le chlorhydrate de chlorpromazine subit diverses réactions chimiques, notamment des réactions d'oxydation, de réduction et de substitution.
Réactifs et conditions courantes
Oxydation : Le chlorhydrate de chlorpromazine peut être oxydé en utilisant des réactifs tels que le permanganate de potassium ou le peroxyde d'hydrogène en milieu acide.
Réduction : La réduction peut être réalisée en utilisant des réactifs tels que l'hydrure de lithium et d'aluminium ou le borohydrure de sodium.
Substitution : Les réactions de substitution impliquent souvent des nucléophiles tels que les ions hydroxyde ou les amines en milieu basique.
Principaux produits
Les principaux produits formés à partir de ces réactions comprennent le sulfoxyde de chlorpromazine (oxydation), la chlorpromazine réduite (réduction) et divers dérivés substitués (substitution) .
Applications de recherche scientifique
Le chlorhydrate de chlorpromazine a une large gamme d'applications de recherche scientifique :
Industrie : Utilisé dans la formulation de produits pharmaceutiques, notamment des granules à libération prolongée et des comprimés à dissolution rapide
Mécanisme d'action
Le chlorhydrate de chlorpromazine agit principalement comme un antagoniste des récepteurs de la dopamine. Il se lie et bloque les récepteurs de la dopamine dans le cerveau, en particulier les récepteurs D2, réduisant l'activité des voies de signalisation de la dopamine. Cette action est considérée comme étant responsable de ses effets antipsychotiques. De plus, le chlorhydrate de chlorpromazine présente des propriétés anti-sérotoninergiques et antihistaminiques, ce qui contribue à son profil thérapeutique global .
Comparaison Avec Des Composés Similaires
Le chlorhydrate de chlorpromazine appartient à la classe des antipsychotiques phénothiaziniques. Des composés similaires comprennent :
Thioridazine : Un dérivé de la phénothiazine présentant des propriétés antipsychotiques similaires, mais un profil d'effets secondaires différent.
Fluphénazine : Un antipsychotique phénothiazinique plus puissant utilisé pour le traitement de la schizophrénie.
Le chlorhydrate de chlorpromazine est unique en raison de ses larges applications thérapeutiques, notamment son utilisation dans le traitement des problèmes de comportement graves chez les enfants et son efficacité pour contrôler les nausées et les vomissements .
Activité Biologique
Chlorpromazine hydrochloride is a first-generation antipsychotic medication primarily used in the treatment of schizophrenia and other psychotic disorders. It was the first antipsychotic drug introduced in the 1950s and remains a cornerstone in psychiatric treatment. This article delves into its biological activity, pharmacological properties, and associated clinical findings, supported by data tables and case studies.
Pharmacological Mechanism
Chlorpromazine acts as a dopamine receptor antagonist , primarily targeting the D2 subtype, but it also interacts with various other neurotransmitter systems. Its broad receptor profile includes:
- Dopaminergic receptors : Antagonizes D1, D2, D3, and D4 receptors, leading to reduced dopaminergic activity, which is beneficial for alleviating psychotic symptoms.
- Serotonergic receptors : Blocks 5-HT1 and 5-HT2 receptors, contributing to its anxiolytic and antidepressant effects while potentially causing weight gain and sedation.
- Histaminergic receptors : Antagonism of H1 receptors results in sedation and antiemetic effects.
- Adrenergic receptors : Exhibits antiadrenergic properties, leading to hypotension and reflex tachycardia.
- Muscarinic receptors : Causes anticholinergic side effects such as dry mouth and constipation.
This multi-receptor interaction is often described as making chlorpromazine a "dirty drug," as it can lead to diverse therapeutic effects as well as adverse reactions .
Table 1: Receptor Binding Affinity of Chlorpromazine
| Receptor Type | Action | Effects |
|---|---|---|
| D1 | Antagonist | Reduced dopaminergic activity |
| D2 | Antagonist | Alleviation of psychotic symptoms |
| 5-HT1/5-HT2 | Antagonist | Anxiolytic effects; potential weight gain |
| H1 | Antagonist | Sedation; antiemetic effects |
| Alpha-adrenergic | Antagonist | Hypotension; reflex tachycardia |
| Muscarinic | Antagonist | Anticholinergic side effects |
Case Study 1: Chlorpromazine-Induced Venous Thromboembolism
A notable case report documented a patient treated with chlorpromazine for acute mania who developed pulmonary embolism (PE). The patient exhibited severe agitation requiring high doses of chlorpromazine. Following four days of treatment, she experienced respiratory distress and was diagnosed with PE. This case highlights the potential risks associated with chlorpromazine, including venous thromboembolism (VTE), particularly in patients with prolonged immobility .
Case Study 2: Chlorpromazine in Terminal Care
In another study involving terminally ill patients, chlorpromazine was administered to manage restlessness and dyspnea. Dosages ranged from 13 to 150 mg/day intravenously or 12 to 300 mg/day rectally over a mean duration of two days. The results indicated effective symptom management without significant adverse effects, suggesting chlorpromazine's utility in palliative care settings .
Table 2: Summary of Clinical Findings
| Study Type | Findings |
|---|---|
| Venous Thromboembolism | Association with high-dose chlorpromazine use |
| Terminal Care | Effective in managing symptoms without major side effects |
Toxicity and Carcinogenicity Studies
Research into the toxicity of chlorpromazine has shown that it is not carcinogenic at tolerated doses. A study using p53 heterozygous mice indicated no significant increase in tumor incidence after administration of chlorpromazine over an extended period. However, there were observations of minimal uterine and ovarian atrophy at higher doses .
Table 3: Toxicity Study Results
| Dose (mg/kg) | Observations |
|---|---|
| 10 | No tumors detected |
| 20 | High mortality due to sedative effects |
| 40 | Poor tolerance observed |
Propriétés
IUPAC Name |
3-(2-chlorophenothiazin-10-yl)-N,N-dimethylpropan-1-amine;hydrochloride | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C17H19ClN2S.ClH/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20;/h3-4,6-9,12H,5,10-11H2,1-2H3;1H | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
FBSMERQALIEGJT-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN(C)CCCN1C2=CC=CC=C2SC3=C1C=C(C=C3)Cl.Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C17H19ClN2S.ClH, C17H20Cl2N2S | |
| Record name | CHLORPROMAZINE HYDROCHLORIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20027 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID7024827 | |
| Record name | Chlorpromazine hydrochloride | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7024827 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
355.3 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Chlorpromazine hydrochloride appears as white or creamy-white odorless crystalline powder with very bitter taste. pH (5% aqueous solution) 4.0-5.5. pH (10% aqueous solution) 4-5. (NTP, 1992) | |
| Record name | CHLORPROMAZINE HYDROCHLORIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20027 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
Solubility |
greater than or equal to 100 mg/mL at 75 °F (NTP, 1992) | |
| Record name | CHLORPROMAZINE HYDROCHLORIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20027 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
CAS No. |
69-09-0 | |
| Record name | CHLORPROMAZINE HYDROCHLORIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20027 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Chlorpromazine hydrochloride | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=69-09-0 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Chlorpromazine hydrochloride [USP:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000069090 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Chlorpromazine hydrochloride | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=17479 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | 10H-Phenothiazine-10-propanamine, 2-chloro-N,N-dimethyl-, hydrochloride (1:1) | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | Chlorpromazine hydrochloride | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7024827 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Chlorpromazine hydrochloride | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.638 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CHLORPROMAZINE HYDROCHLORIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/9WP59609J6 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Melting Point |
378 to 385 °F (decomposes) (NTP, 1992) | |
| Record name | CHLORPROMAZINE HYDROCHLORIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20027 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of Chlorpromazine Hydrochloride?
A1: this compound primarily acts as a dopamine antagonist, blocking the action of dopamine in the brain. This is thought to be responsible for its antipsychotic effects. []
Q2: How does this compound affect prolactin levels?
A2: this compound can stimulate prolactin release in rats, with peak levels observed between 30 and 90 minutes after administration. This effect is dose-dependent and correlates with the drug's antipsychotic potency in humans. []
Q3: Does this compound impact bile secretion?
A3: Yes, this compound can induce cholestasis, a condition characterized by reduced bile flow. This effect is likely due to the drug's ability to form insoluble complexes with bile salts, leading to their precipitation and reduced secretion. [] Studies in Rhesus monkeys demonstrate that this effect is dose-dependent and reversible, with bile flow returning to normal as drug concentrations decrease. []
Q4: What is the molecular formula and weight of this compound?
A4: The molecular formula of this compound is C17H19ClN2S·HCl, and its molecular weight is 355.33 g/mol.
Q5: Does this compound interact with lipids?
A5: Yes, being an amphiphilic molecule, this compound can interact with both polar and non-polar molecules. Studies show that it can interact with bile lipids, forming insoluble complexes with bile salts and solubilizing membrane phospholipids like phosphatidylserine and phosphatidylcholine. []
Q6: How does the structure of this compound contribute to its interaction with bile salts?
A6: this compound is amphiphilic due to its polar tertiary amine group linked to a hydrophobic tricyclic ring system by a short paraffin chain. This structure allows it to interact with both the hydrophilic and hydrophobic regions of bile salts, leading to complex formation and precipitation. []
Q7: Does the sugar-coating on this compound tablets interfere with content determination?
A7: No, studies have shown that the sugar-coating does not interfere with UV spectrophotometric determination of this compound content in tablets. Therefore, shelling the sugar-coat is unnecessary for accurate analysis. []
Q8: What is the impact of this compound on regional blood flow in acute cerebral infarction patients?
A8: Studies show that this compound, particularly when combined with ozagrel, can significantly improve regional blood flow speed in acute cerebral infarction patients. This combination also effectively reduces blood viscosity. []
Q9: What effect does this compound have on diffuse large B lymphoma cells?
A9: In vitro studies show that this compound can inhibit the proliferation of diffuse large B lymphoma cells, potentially by promoting the expression of S1PR2. It also induces apoptosis and promotes G1 cell cycle arrest in these cells. []
Q10: Has this compound shown efficacy in treating intractable hiccups?
A10: Yes, clinical observations suggest that this compound is effective in treating intractable hiccups, showing positive results through various administration routes like acupoint injection, nasal mucosa medication, and combined with traditional Chinese medicine. [, , ]
Q11: What analytical methods are commonly employed for this compound quantification?
A11: Several analytical methods are used for this compound quantification, including:
- Spectrophotometry: This method relies on the drug's absorbance at specific wavelengths, but it can be influenced by excipients or degradation products. [, , , , , , , , ]
- Spectrofluorimetry: This method measures the drug's fluorescence intensity, offering high sensitivity and a low detection limit. [, ]
- Chemiluminescence: This technique exploits the light emitted during a chemical reaction involving the drug, offering high sensitivity. [, ]
- High-performance liquid chromatography (HPLC): This method separates the drug from other components in a sample before detection, offering high selectivity and sensitivity. [, ]
- Ultra performance liquid chromatography-tandem mass spectrometry (UPLC/MS/MS): This method combines the separation power of HPLC with the sensitivity and selectivity of mass spectrometry. []
- Flow injection analysis: This technique automates sample injection and analysis, improving speed and precision. [, , , ]
- Resonance scattering spectrometry: This method measures the scattering of light by nanoparticles formed upon reaction with the drug, offering high sensitivity and selectivity. []
Q12: Are there specific challenges in analyzing this compound in biological samples?
A12: Yes, biological matrices can introduce interferences, requiring extraction and cleanup procedures before analysis. Methods like UPLC/MS/MS are particularly useful for analyzing trace levels in complex matrices like animal tissues. []
Q13: How do analytical methods ensure accurate and reliable determination of this compound?
A13: Analytical methods undergo validation to ensure their accuracy, precision, and specificity for this compound determination. This involves assessing parameters like linearity, range, limit of detection, limit of quantification, and recovery. [, , , , , , , , , ]
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.















