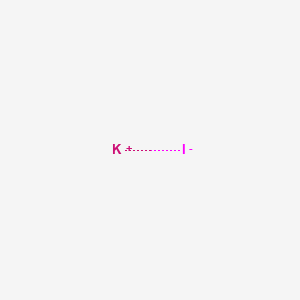
Iodure de potassium
Vue d'ensemble
Description
L'iodure de potassium est un composé inorganique de formule chimique KI. Il s'agit d'un sel métallique-halogénure présentant une liaison ionique entre le cation potassium (K⁺) et l'anion iodure (I⁻). Ce composé se présente sous forme de cristaux cubiques incolores à blancs, de poudre ou de granulés blancs. L'this compound est largement utilisé en médecine, en protection contre les rayonnements et comme complément alimentaire .
Mécanisme D'action
Target of Action
Potassium iodide primarily targets the thyroid gland . It is used as an oral antithyroid agent for the prevention of radioactive iodine uptake into the thyroid gland during a nuclear radiation emergency . It can also be used for the treatment of hyperthyroidism and thyrotoxicosis .
Biochemical Pathways
Iodine is a component of biochemical pathways in organisms from all biological kingdoms, suggesting its fundamental significance throughout the evolutionary history of life . In vertebrate biology, iodine’s primary function is as a constituent of the thyroid hormones, thyroxine (T4) and triiodothyronine (T3). These hormones are made from addition-condensation products of the amino acid tyrosine, and are stored prior to release in an iodine-containing protein called thyroglobulin .
Pharmacokinetics
Potassium iodide is adequately absorbed from the gastrointestinal tract . Its effects on thyroid function are usually observed within 24 hours after administration and are maximal after 10-15 days of continuous therapy . It is cleared from the plasma by renal excretion or thyroid uptake .
Result of Action
The result of potassium iodide’s action is the prevention of radioactive iodine uptake into the thyroid gland during a nuclear radiation emergency, thereby reducing the risk of thyroid cancer . It also inhibits the secretion of thyroid hormone, fosters colloid accumulation in thyroid follicles, and reduces thyroid gland vascularity .
Applications De Recherche Scientifique
Medical Applications
Thyroid Protection in Radiation Emergencies
Potassium iodide is primarily known for its role in protecting the thyroid gland from radioactive iodine exposure during nuclear emergencies. By saturating the thyroid with non-radioactive iodine, KI prevents the uptake of harmful isotopes, thereby reducing the risk of thyroid cancer and other related disorders. This application was notably utilized during the Chernobyl disaster and continues to be recommended in emergency preparedness plans for nuclear incidents .
Treatment of Thyroid Disorders
In clinical settings, potassium iodide is used as an adjunct treatment for hyperthyroidism and thyrotoxicosis. It helps to decrease thyroid hormone production and can be administered preoperatively to induce thyroid involution before surgery . The compound's ability to inhibit the release of T4 and T3 hormones makes it effective in managing thyroid storm conditions .
Dermatological Uses
Potassium iodide has been employed in dermatology for its immunomodulatory properties. It is used in treating various skin conditions due to its anti-inflammatory effects, particularly in diseases where neutrophil activity is implicated . A saturated solution of KI can serve as an expectorant for respiratory tract infections, aiding in mucus clearance .
Agricultural Applications
Iodization of Salt
KI is widely used as a food additive to iodize table salt, an essential public health measure aimed at preventing iodine deficiency disorders in populations with low dietary iodine intake. This practice has significantly improved iodine levels in many regions globally .
Animal Feed Supplement
In addition to human consumption, potassium iodide is also added to animal feeds as a dietary supplement to ensure adequate iodine intake in livestock, which is crucial for maintaining overall health and productivity .
Chemical Synthesis Applications
Reagent in Organic Chemistry
Potassium iodide serves as a critical reagent in various chemical reactions. Its nucleophilic properties allow it to participate in substitution reactions and facilitate the synthesis of aryl iodides through methods such as the Sandmeyer reaction . KI is also utilized in quantitative chemical analyses, including iodometric titrations and spectroscopy .
Photographic Emulsions
In photography, potassium iodide is a key component in the production of silver iodide, which is essential for light-sensitive photographic emulsions. This application underscores KI's significance beyond medicinal uses into industrial processes .
Environmental Applications
Water Purification
KI acts as a sanitizing agent in water treatment processes. Its antimicrobial properties enable it to effectively disinfect drinking water and sanitize food processing equipment, contributing to public health safety standards .
Data Table: Summary of Applications
| Application Area | Specific Uses |
|---|---|
| Medical | Thyroid protection during radiation emergencies |
| Treatment of hyperthyroidism | |
| Dermatological treatments | |
| Agricultural | Iodization of table salt |
| Dietary supplement in animal feeds | |
| Chemical Synthesis | Reagent in organic reactions (e.g., Sandmeyer reaction) |
| Quantitative analyses (iodometric titrations) | |
| Environmental | Water purification and disinfection |
Case Studies
- Chernobyl Nuclear Disaster (1986) : The administration of potassium iodide to residents near the Chernobyl Nuclear Power Plant significantly reduced the incidence of thyroid cancer among exposed populations by blocking radioactive iodine uptake .
- Iodine Deficiency Disorders (IDD) : A study conducted in various regions revealed that mandatory salt iodization programs using potassium iodide led to a dramatic decrease in IDD prevalence, demonstrating the effectiveness of KI as a public health intervention .
- Antimicrobial Photodynamic Therapy (aPDT) : Research has shown that adding potassium iodide enhances the efficacy of certain photosensitizers against multidrug-resistant bacteria, indicating its potential role in novel therapeutic approaches against infections .
Analyse Biochimique
Biochemical Properties
Potassium iodide plays a significant role in biochemical reactions. It is used in colorimetric assays to detect hydrogen peroxide concentrations in leaf extracts . The method is based on the oxidation of potassium iodide .
Molecular Mechanism
The molecular mechanism of potassium iodide primarily involves its reaction with hydrogen peroxide. In this reaction, potassium iodide is oxidized, indicating that it may interact with other biomolecules in a similar manner .
Metabolic Pathways
Potassium iodide is involved in the metabolic pathway of hydrogen peroxide detection, where it acts as a chromophore and undergoes oxidation .
Méthodes De Préparation
Voies synthétiques et conditions réactionnelles : L'iodure de potassium est généralement préparé en faisant réagir l'iode avec de l'hydroxyde de potassium. La réaction implique la dissolution de l'iode dans l'eau, puis l'ajout d'une solution d'hydroxyde de potassium. Le mélange est chauffé pour compléter la réaction, ce qui conduit à la formation d'this compound et d'eau :
I2+2KOH→2KI+H2O
Méthodes de production industrielle : Dans les milieux industriels, l'this compound est produit en ajoutant de l'iode à une solution d'hydroxyde de potassium. Le mélange réactionnel est ensuite chauffé, et la solution résultante est évaporée pour faire cristalliser l'this compound. Les cristaux sont ensuite filtrés, lavés et séchés pour obtenir le produit final {_svg_2}.
Types de réactions :
- L'this compound peut être oxydé en iode par divers agents oxydants. Par exemple:
Oxydation : 2KI+Cl2→2KCl+I2
Réduction : L'this compound peut agir comme agent réducteur dans certaines réactions.
Substitution : L'this compound est utilisé dans les réactions de substitution nucléophile pour introduire des ions iodure dans les molécules organiques.
Réactifs et conditions courantes :
Agents oxydants : Chlore, brome et autres halogènes.
Agents réducteurs : Thiosulfate de sodium, dioxyde de soufre.
Solvants : Eau, éthanol.
Principaux produits :
Iode (I₂) : Formé lors de réactions d'oxydation.
Chlorure de potassium (KCl) : Formé lors de réactions de substitution avec le chlore {_svg_3}
Chimie :
Titrages iodométriques : L'this compound est utilisé comme réactif analytique dans les titrages iodométriques pour déterminer la concentration des agents oxydants.
Synthèse des iodures d'aryle : Il sert de source d'iodure dans la synthèse des iodures d'aryle à partir de sels de diazonium.
Biologie :
Complément nutritionnel : L'this compound est ajouté au sel de table pour prévenir les carences en iode chez l'homme et les animaux.
Protection de la thyroïde : Il est utilisé pour protéger la glande thyroïde de l'iode radioactif lors d'urgences radiologiques.
Médecine :
Traitement de l'hyperthyroïdie : L'this compound est utilisé pour traiter l'hyperthyroïdie en réduisant la production d'hormones thyroïdiennes.
Expectorant : Il aide à briser les mucosités dans les voies respiratoires, ce qui facilite la respiration.
Industrie :
Photographie : L'this compound est utilisé dans la préparation des émulsions photographiques.
Fabrication de colorants : Il est utilisé comme matière première dans la production de colorants.
5. Mécanisme d'action
L'this compound agit principalement dans la glande thyroïde. Il inhibe la synthèse et la libération d'hormones thyroïdiennes, réduit la vascularisation de la glande thyroïde et augmente l'accumulation de colloïde dans les follicules thyroïdiens. Cela se traduit par une glande thyroïde plus ferme avec une taille cellulaire réduite et des taux d'iode lié augmentés. De plus, l'this compound bloque l'absorption de l'iode radioactif par la glande thyroïde, réduisant ainsi le risque de cancer de la thyroïde lors d'une exposition aux rayonnements .
Composés similaires :
Iodate de potassium (KIO₃) : Utilisé comme supplément d'iode dans le sel et pour le blocage de la thyroïde lors d'urgences radiologiques.
Iodure de sodium (NaI) : Utilisé dans des applications similaires à celles de l'this compound, mais avec des propriétés de solubilité et de réactivité différentes.
Iodure de calcium (CaI₂) : Utilisé dans l'alimentation animale et comme source d'iode dans diverses applications
Unicité de l'this compound : L'this compound est unique en raison de sa grande solubilité dans l'eau, ce qui en fait une source efficace d'ions iodure dans diverses applications chimiques et biologiques. Sa capacité à protéger la glande thyroïde de l'iode radioactif et son utilisation dans les titrages iodométriques mettent en évidence sa polyvalence et son importance .
Comparaison Avec Des Composés Similaires
Potassium Iodate (KIO₃): Used as an iodine supplement in salt and for thyroid blocking in radiation emergencies.
Sodium Iodide (NaI): Used in similar applications as potassium iodide but with different solubility and reactivity properties.
Calcium Iodide (CaI₂): Used in animal feed and as a source of iodine in various applications
Uniqueness of Potassium Iodide: Potassium iodide is unique due to its high solubility in water, making it an effective source of iodide ions in various chemical and biological applications. Its ability to protect the thyroid gland from radioactive iodine and its use in iodometric titrations further highlight its versatility and importance .
Activité Biologique
Potassium iodide (KI) is a compound with significant biological activities, particularly in the fields of medicine and microbiology. This article explores its various biological effects, mechanisms of action, clinical applications, and relevant case studies.
Potassium iodide is an inorganic compound that dissociates into potassium ions (K⁺) and iodide ions (I⁻) in solution. The biological activity of KI is primarily attributed to the iodide ion, which plays a crucial role in thyroid hormone synthesis and has antimicrobial properties.
Mechanism of Action:
- Thyroid Function: KI provides iodide necessary for the synthesis of thyroid hormones (T3 and T4). Excess iodide can inhibit thyroid hormone synthesis through the Wolff-Chaikoff effect, where high levels of iodide lead to reduced uptake and organification by the thyroid gland .
- Antimicrobial Activity: KI exhibits broad-spectrum antimicrobial activity. It can generate molecular iodine (I₂) and reactive oxygen species (ROS), which are effective in killing bacteria, particularly during photodynamic therapy (PDT) .
Biological Activities
-
Thyroid Disorders:
- KI is used in treating hyperthyroidism and thyroid storm. Clinical studies indicate that it can reduce in-hospital mortality among patients with Graves' disease experiencing thyroid storm .
- A study involving 11 patients with painless thyroiditis induced by KI showed that all patients returned to euthyroid status after treatment .
-
Antimicrobial Properties:
- KI enhances the efficacy of antimicrobial photodynamic therapy (aPDT). It has been shown to potentiate the killing effects of various photosensitizers on Gram-negative bacteria, such as Acinetobacter baumannii .
- The formation of hypoiodite and molecular iodine during aPDT contributes to its antibacterial activity, demonstrating a potential application in treating infections .
- Radioprotection:
Case Study 1: Potassium Iodide-Induced Painless Thyroiditis
A study documented 11 cases of painless thyroiditis occurring during KI treatment for Graves' disease. The patients exhibited transient hyperthyroidism characterized by low technetium uptake. All but one patient returned to a euthyroid state without requiring additional treatment .
Case Study 2: KI in Thyroid Storm Management
Research analyzing outcomes for patients hospitalized with thyroid storm found that those treated with KI had lower in-hospital mortality rates compared to those who did not receive KI. The study involved 3,188 patients, revealing a significant reduction in mortality among those diagnosed with Graves' disease who received early KI treatment .
Data Summary
Propriétés
IUPAC Name |
potassium;iodide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/HI.K/h1H;/q;+1/p-1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
NLKNQRATVPKPDG-UHFFFAOYSA-M | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
[K+].[I-] | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
KI, IK | |
| Record name | POTASSIUM IODIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/9014 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | potassium iodide | |
| Source | Wikipedia | |
| URL | https://en.wikipedia.org/wiki/Potassium_iodide | |
| Description | Chemical information link to Wikipedia. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID7034836 | |
| Record name | Potassium iodide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7034836 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
166.0028 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Potassium iodide is an odorless white solid. Sinks and mixes with water. (USCG, 1999), Dry Powder; Dry Powder, Liquid; Liquid; Pellets or Large Crystals, Other Solid, Water-soluble, white solid; [CAMEO] Slightly deliquescent; [CHEMINFO] | |
| Record name | POTASSIUM IODIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/9014 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Potassium iodide (KI) | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | Potassium iodide | |
| Source | Haz-Map, Information on Hazardous Chemicals and Occupational Diseases | |
| URL | https://haz-map.com/Agents/2028 | |
| Description | Haz-Map® is an occupational health database designed for health and safety professionals and for consumers seeking information about the adverse effects of workplace exposures to chemical and biological agents. | |
| Explanation | Copyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission. | |
Boiling Point |
Very high (USCG, 1999), 1323 °C | |
| Record name | POTASSIUM IODIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/9014 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | POTASSIUM IODIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/5040 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Solubility |
Slightly soluble in ethanol, 148 g/100 g water at 25 °C, 127.5 g sol in 100 cc water at 0 °C; 1.88 g sol in 100 cc alcohol at 25 °C; 1.31 g sol in 100 cc acetone at 25 °C; sol in ether, ammonia, 1 gram dissolves in 0.7 mL water, 0.5 mL boiling water; 51 mL absolute ethanol; 22 mL alcohol; 8 mL methanol; 75 mL acetone; 2 mL glycerol; about 2.5 mL glycol | |
| Record name | POTASSIUM IODIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/5040 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Density |
3.13 at 59 °F (USCG, 1999) - Denser than water; will sink, 3.12 g/cu cm | |
| Record name | POTASSIUM IODIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/9014 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | POTASSIUM IODIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/5040 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
In hyperthyroid patients, potassium iodide produces rapid remission of symptoms by inhibiting the release of thyroid hormone into the circulation. The effects of potassium iodide on the thyroid gland include reduction of vascularity, a firming of the glandular tissue, shrinkage of the size of individual cells, reaccumulation of colloid in the follicles, and increases in bound iodine. These actions may facilitate thyroidectomy when the medication is given prior to surgery., When administered prior to and following administration of radioactive isotopes and in radiation emergencies involving the release of radioactive iodine, potassium iodide protects the thyroid gland by blocking the thyroidal uptake of radioactive isotopes of iodine., The mechanism of action of potassium iodide's antifungal activity against Sporothix schenckii has not been determined. Potassium iodide does not appear to increase monocyte or neutrophil killing of S. schenckii in in vitro studies. However, exposure of the yeast form of S. schenckii to various concentrations of iodine (iodine and potassium iodide solution) has resulted in rapid cell destruction., Potassium iodide is thought to act as an expectorant by increasing respiratory tract secretions and thereby decreasing the viscosity of mucous ..., When potassium iodide is administered simultaneously with radiation exposure, the protectant effect is approximately 97%. Potassium iodide given 12 and 24 hours before exposure yields a 90% and 70% protectant effect, respectively. However, potassium iodide administered 1 and 3 hours after exposure results in an 85% and 50% protectant effect, respectively. Potassium iodide administered more than 6 hours after exposure is thought to have a negligible protectant effect. | |
| Record name | POTASSIUM IODIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/5040 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Impurities |
Impurities: sulfates and heavy metals, Typically less than 1 ppm H2O-OH in 99.9995% purity, ultradry grades | |
| Record name | POTASSIUM IODIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/5040 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Colorless or white, cubical crystals, white granules, or powder, HEXAHEDRAL CRYSTALS, EITHER TRANSPARENT OR SOMEWHAT OPAQUE | |
CAS No. |
7681-11-0 | |
| Record name | POTASSIUM IODIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/9014 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Potassium iodide | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=7681-11-0 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Potassium iodide [USP:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0007681110 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Potassium Iodide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB06715 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | potassium iodide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=77362 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Potassium iodide (KI) | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | Potassium iodide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7034836 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Potassium iodide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.028.782 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | Potassium iodide | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/1C4QK22F9J | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | POTASSIUM IODIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/5040 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Melting Point |
1258 °F (USCG, 1999), 681 °C | |
| Record name | POTASSIUM IODIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/9014 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | POTASSIUM IODIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/5040 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Q1: How does potassium iodide interact with the thyroid gland?
A1: Potassium iodide provides the essential nutrient iodine, which the thyroid gland actively absorbs to produce the hormones thyroxine (T4) and triiodothyronine (T3). In situations of radioactive iodine exposure, potassium iodide saturates the thyroid with stable iodine, blocking the uptake of the radioactive form and reducing the risk of thyroid cancer. []
Q2: Can potassium iodide induce hyperthyroidism?
A2: Yes, in some cases, particularly in individuals with pre-existing thyroid conditions or those sensitive to iodine, potassium iodide administration can lead to hyperthyroidism. This was observed in three geriatric patients receiving potassium iodide during a 125I-fibrinogen test. []
Q3: What are the recommended daily allowances for iodine intake?
A3: The recommended daily allowances (RDA) for iodine intake are 150 μg in adults, 220–250 μg in pregnant women, and 250–290 μg in breastfeeding women. [] The American Thyroid Association (ATA) recommends that women take a multivitamin containing 150 μg of iodine daily in the form of potassium iodide (KI) during preconception, pregnancy, and lactation to meet these needs. []
Q4: What is the effect of potassium iodide on cathepsins in rat thyroid cells?
A6: Research using the FRTL thyroid cell line shows that high concentrations of potassium iodide (50 mmol/L) significantly decrease the activity of cathepsins B and D. These enzymes play a role in thyroid hormone synthesis and release, suggesting iodine excess might affect thyroid function through this mechanism. []
Q5: What is the molecular formula and weight of potassium iodide?
A5: The molecular formula of potassium iodide is KI, and its molecular weight is 166.00 g/mol.
Q6: Can potassium iodide be used as a photostabilizer?
A9: Yes, potassium iodide solutions, which tend to turn yellow upon light exposure due to iodine liberation, can be stabilized using compounds like DL-methionine and thiourea. These compounds prevent color change even under intense light exposure for extended periods. []
Q7: How does potassium iodide function as a catalyst?
A11: Potassium iodide can act as a catalyst in various reactions. For instance, it catalyzes the synthesis of 1,3-dioxolan-2-one derivatives from epoxides and carbon dioxide, even at relatively low temperatures (60°C). []
Q8: Is potassium iodide truly a catalyst in the decomposition of hydrogen peroxide?
A12: Contrary to common belief, experimental evidence suggests that potassium iodide is not a true catalyst in hydrogen peroxide decomposition. While it accelerates the reaction, it also undergoes chemical changes, as evidenced by the solution turning yellow due to iodine formation and changes in pH, conductivity, and iodide ion concentration. []
Q9: Can potassium iodide be used in conjunction with biomass for CO2 conversion?
A13: Yes, research shows that dewaxed sugarcane bagasse combined with potassium iodide can act as an effective catalytic system for converting CO2 to cyclic carbonates. The hydroxyl groups in the biomass play a crucial role in this process by activating the epoxide ring opening. []
Q10: Have computational methods been used to study potassium iodide?
A14: Yes, molecular beam scattering data has been used to create highly accurate excited-state potential energy surfaces for potassium iodide, furthering the understanding of its spectroscopic properties and behavior in excited states. []
Q11: Does the counterion to iodide affect iodine absorption?
A15: Yes, studies comparing potassium iodide (KI) and sodium iodide (NaI) in rabbits showed that KI resulted in peak blood iodine levels being maintained for a longer duration compared to NaI. This suggests that the counterion can influence the pharmacokinetics of iodide. []
Q12: How can the stability of potassium iodide formulations be improved?
A16: Potassium iodide jellied pharmaceutical compositions have been developed to improve storage stability and dissolution, allowing for easier medication. These formulations typically include gelling agents and dispersion media to achieve the desired properties. []
Q13: How is iodine absorbed and distributed in the body after potassium iodide administration?
A17: Following oral administration, potassium iodide is readily absorbed in the gastrointestinal tract. Iodine is then distributed throughout the body, with the thyroid gland actively accumulating it for hormone synthesis. Excess iodine is primarily excreted in urine. []
Q14: How does the absorption of iodine from potassium iodide compare to that of iodine from kombu?
A18: Studies in rats have shown that the absorption of iodine from potassium iodide is significantly higher compared to iodine from kombu, a type of seaweed. This difference was observed by comparing serum and tissue iodine concentrations in rats fed diets supplemented with either potassium iodide or kombu powder. []
Q15: Can potassium iodide be used to treat haemoptysis caused by aspergillomas?
A19: Yes, in cases where surgery is not an option, intracavitary instillation of sodium or potassium iodide has been shown to be effective in stopping life-threatening haemoptysis from aspergillomas. []
Q16: What are the potential risks of excess iodine ingestion?
A20: Ingesting iodine in amounts exceeding the tolerable upper limits (1100 μg per day) can lead to thyroid dysfunction, particularly in susceptible individuals like infants, pregnant and breastfeeding women, the elderly, and those with pre-existing thyroid conditions. [] High iodine intake can cause effects ranging from goiter to hypothyroidism or hyperthyroidism. []
Q17: What analytical methods are used to determine iodide levels?
A21: Several methods are available for determining iodide levels, including volumetric titration methods like the Sadusk-Ball procedure, which is particularly useful for analyzing iodized salt. [] Other methods might include ion chromatography or spectrophotometry after appropriate chemical derivatization.
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.















