Nystatin
Vue d'ensemble
Description
La nystatine est un antifongique polyénique qui présente une activité fongicide et fongistatique à large spectre contre une variété de levures et de champignons, notamment les espèces Candida . Elle a été découverte en 1950 par Rachel Fuller Brown et Elizabeth Lee Hazen et est produite par la bactérie Streptomyces noursei . La nystatine est utilisée pour traiter les infections mycotiques cutanées, mucocutanées et gastro-intestinales .
Méthodes De Préparation
La nystatine est généralement extraite du bouillon de fermentation de Streptomyces noursei en utilisant un solvant organique soluble dans l'eau. Le composé est ensuite purifié et cristallisé . La production industrielle de nystatine implique une série d'étapes, notamment la fermentation, l'extraction, la purification et la cristallisation afin d'obtenir des cristaux de nystatine à haut rendement et de haute pureté .
Analyse Des Réactions Chimiques
La nystatine subit diverses réactions chimiques, notamment l'oxydation et la réduction. Le composé est amphotère en raison de la présence de groupes carboxyle et amino . Elle est pratiquement insoluble dans l'eau mais légèrement soluble dans les solvants organiques polaires . La solubilité de la nystatine augmente dans les systèmes aqueux-organiques, la plus grande solubilité étant atteinte dans des solutions contenant 30 à 40 % d'eau .
Applications de la recherche scientifique
La nystatine est largement utilisée dans la recherche scientifique, en particulier dans les domaines de la chimie, de la biologie, de la médecine et de l'industrie. En médecine, elle est utilisée pour traiter la candidose orale, la candidose intestinale et la candidose anale . Elle est également utilisée par voie topique pour le traitement de la candidose vulvovaginale et d'autres infections cutanées à Candida . De plus, la nystatine est utilisée en association avec d'autres médicaments pour traiter les infections mixtes . En recherche scientifique, la nystatine est utilisée pour étudier les effets des agents antifongiques sur les membranes cellulaires fongiques et pour étudier les mécanismes moléculaires des infections fongiques .
Mécanisme d'action
La nystatine exerce ses effets antifongiques en se liant à l'ergostérol, un composant clé des membranes cellulaires fongiques . Cette liaison provoque la formation de pores dans la membrane, ce qui entraîne une fuite d'ions potassium et d'autres contenus cellulaires, entraînant finalement la mort cellulaire . Le mécanisme d'action de la nystatine est complexe et dépend de la présence et du type de stérols membranaires . La formation et la stabilisation des pores induits par la nystatine sont influencées par les propriétés biophysiques et la composition lipidique de la membrane .
Applications De Recherche Scientifique
Treatment of Oral Candidiasis
Nystatin is commonly used for treating oral candidiasis, especially in immunocompromised patients. A systematic review indicated that this compound pastilles demonstrated a higher clinical cure rate (87%) compared to this compound suspension (80%) in patients with denture stomatitis .
Efficacy Comparison Table:
| Treatment Type | Clinical Cure Rate (%) | Mycological Cure Rate (%) |
|---|---|---|
| This compound Pastilles | 87 | Not specified |
| This compound Suspension | 80 | Not specified |
| Fluconazole | 88 | Not specified |
Prophylaxis in Immunocompromised Patients
This compound has been investigated for prophylactic use in severely immunodepressed patients, such as those undergoing chemotherapy or organ transplantation. However, studies have shown that its efficacy is often inferior to fluconazole, with this compound demonstrating no significant advantage over placebo in preventing fungal colonization .
Prophylaxis Effectiveness Table:
| Study Group | This compound Efficacy | Fluconazole Efficacy | Placebo Efficacy |
|---|---|---|---|
| Acute leukemia patients | No significant difference | Superior | No significant difference |
| Solid cancer patients | No significant difference | Superior | No significant difference |
| Bone marrow transplant recipients | No significant difference | Superior | No significant difference |
Treatment of Cutaneous Infections
This compound is also effective against cutaneous candidiasis and other superficial fungal infections. In cases where patients exhibit hypersensitivity to fungi, this compound has shown superior efficacy compared to placebo in reducing localized and systemic symptoms .
Case Studies
-
Oral Candidiasis in Cancer Patients
A study involving hospitalized cancer patients found that the combination of this compound suspension and pastilles resulted in clinical and mycological cures in 87.5% and 66% of cases, respectively . This highlights this compound's effectiveness in vulnerable populations. -
Fungal Hypersensitivity
In a randomized trial with polysymptomatic patients suspected of having fungus hypersensitivity, this compound significantly reduced overall symptom scores compared to placebo (P < 0.003). This suggests potential benefits beyond traditional antifungal applications .
Safety and Side Effects
While this compound is generally well-tolerated, some common side effects include gastrointestinal discomfort and a poor taste associated with oral formulations. These adverse effects can impact patient adherence to treatment regimens .
Mécanisme D'action
Nystatin exerts its antifungal effects by binding to ergosterol, a key component of fungal cell membranes . This binding causes the formation of pores in the membrane, leading to the leakage of potassium ions and other cellular contents, ultimately resulting in cell death . This compound’s mechanism of action is complex and depends on the presence and type of membrane sterols . The formation and stabilization of this compound-induced pores are influenced by the biophysical properties and lipid composition of the membrane .
Comparaison Avec Des Composés Similaires
La nystatine est souvent comparée à d'autres agents antifongiques tels que le fluconazole et l'amphotéricine B. Bien que la nystatine soit efficace pour traiter les infections fongiques localisées, elle ne convient pas à une utilisation systémique en raison de sa toxicité significative lorsqu'elle est administrée par voie parentérale . Le fluconazole, quant à lui, est efficace pour les infections fongiques systémiques mais peut avoir des effets secondaires tels que des maux de tête, des nausées et des douleurs abdominales . L'amphotéricine B est étroitement liée à la nystatine en structure et possède une activité antifongique plus large, mais elle présente également un profil de toxicité plus élevé . D'autres composés similaires comprennent de nouveaux dérivés de thiazole synthétisés, qui ont montré une activité antifongique prometteuse contre les espèces Candida .
La capacité unique de la nystatine à se lier à l'ergostérol et à perturber les membranes cellulaires fongiques en fait un agent antifongique précieux, en particulier pour traiter les infections localisées .
Activité Biologique
Nystatin is a polyene antifungal agent primarily used to treat fungal infections, particularly those caused by Candida species. Its biological activity is characterized by its mechanism of action, efficacy in clinical applications, and safety profile. This article delves into these aspects, supported by research findings, data tables, and case studies.
This compound exerts its antifungal effects through a unique mechanism involving the binding to ergosterol, a key component of fungal cell membranes. This interaction leads to the formation of pores in the membrane, disrupting its integrity and causing leakage of intracellular contents. The selectivity for fungal cells over mammalian cells is attributed to this compound's higher affinity for ergosterol compared to cholesterol found in human cells .
Key Findings on Mechanism
- Pore Formation : this compound forms membrane-spanning channels that increase permeability, allowing ions and small molecules to leak out, which disrupts essential cellular functions .
- Killing Kinetics : Studies show that this compound exhibits rapid killing kinetics against Candida albicans, with significant reductions in colony-forming units (CFUs) observed within hours at effective concentrations .
Efficacy in Clinical Applications
This compound is widely used for treating various fungal infections, particularly oral candidiasis and cutaneous candidiasis. Its formulations include suspensions and pastilles, each demonstrating varying degrees of efficacy.
Clinical Studies Overview
- Oral Candidiasis : A systematic review indicated that this compound pastilles were significantly more effective than placebo for denture stomatitis. However, this compound suspension showed inferior efficacy compared to fluconazole in treating oral candidiasis in vulnerable populations such as infants and HIV/AIDS patients .
- Cutaneous Candidiasis : Historical studies have shown this compound ointment to be effective against skin lesions caused by Candida, with significant clinical response rates compared to placebo .
Efficacy Data Table
| Formulation | Condition | Efficacy (%) | Comparison Drug |
|---|---|---|---|
| This compound Pastille | Denture Stomatitis | 79.6% - 87.5% | Placebo |
| This compound Suspension | Oral Candidiasis | 9% - 63.5% | Fluconazole |
| This compound Ointment | Cutaneous Candidiasis | 78% (clearing rate) | Placebo |
Safety Profile
This compound is generally considered safe with minimal systemic absorption when administered orally or topically. Common adverse effects include gastrointestinal disturbances and a poor taste associated with oral formulations . Notably, high concentrations can lead to cytotoxicity in mammalian cells; however, formulations like this compound-Intralipid have shown reduced toxicity while maintaining antifungal activity .
Case Studies
Several case studies illustrate the effectiveness of this compound in treating specific patient populations:
- Infants with Oral Thrush : In cases involving infants with oral thrush, this compound was administered as a swish-and-swallow treatment, showing significant clinical improvements by day four post-treatment .
- Cancer Patients : A study involving cancer patients demonstrated a clinical cure rate of 87.5% when using a combination of this compound suspension and pastilles for oral candidiasis .
Propriétés
IUPAC Name |
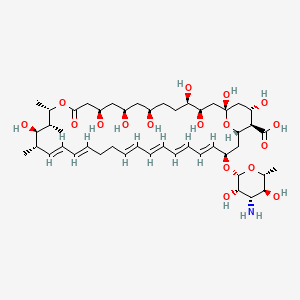
(1S,3R,4R,7R,9R,11R,15S,16R,17R,18S,19E,21E,25E,27E,29E,31E,33R,35S,36R,37S)-33-[(2R,3S,4S,5S,6R)-4-amino-3,5-dihydroxy-6-methyloxan-2-yl]oxy-1,3,4,7,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-14,39-dioxabicyclo[33.3.1]nonatriaconta-19,21,25,27,29,31-hexaene-36-carboxylic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C47H75NO17/c1-27-17-15-13-11-9-7-5-6-8-10-12-14-16-18-34(64-46-44(58)41(48)43(57)30(4)63-46)24-38-40(45(59)60)37(54)26-47(61,65-38)25-36(53)35(52)20-19-31(49)21-32(50)22-33(51)23-39(55)62-29(3)28(2)42(27)56/h5-6,8,10-18,27-38,40-44,46,49-54,56-58,61H,7,9,19-26,48H2,1-4H3,(H,59,60)/b6-5+,10-8+,13-11+,14-12+,17-15+,18-16+/t27-,28-,29-,30+,31+,32+,33+,34-,35+,36+,37-,38-,40+,41-,42+,43+,44-,46-,47+/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
VQOXZBDYSJBXMA-NQTDYLQESA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1C=CC=CCCC=CC=CC=CC=CC(CC2C(C(CC(O2)(CC(C(CCC(CC(CC(CC(=O)OC(C(C1O)C)C)O)O)O)O)O)O)O)C(=O)O)OC3C(C(C(C(O3)C)O)N)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C[C@H]1/C=C/C=C/CC/C=C/C=C/C=C/C=C/[C@@H](C[C@H]2[C@@H]([C@H](C[C@](O2)(C[C@H]([C@@H](CC[C@H](C[C@H](C[C@H](CC(=O)O[C@H]([C@@H]([C@@H]1O)C)C)O)O)O)O)O)O)O)C(=O)O)O[C@H]3[C@H]([C@H]([C@@H]([C@H](O3)C)O)N)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C47H75NO17 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID80872323 | |
| Record name | (7R,10R)-8,9-Dideoxy-28,29-dihydro-7,10-dihydroxyamphotericin B | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID80872323 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
926.1 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
MW: 926.12 /Form not specified/, Mg/ml at about 28 °C: methanol 11.2, ethanol 1.2, chloroform 0.48, carbon tetrachloride 1.23, benzene 0.28, toluene 0.285, acetone 0.390, ethyl acetate 0.75, ethylene glycol 8.75, Insol in ether, In water, 3.60X10+2 mg/L at 24 °C | |
| Record name | NYSTATIN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3138 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
Nystatin exerts its antifungal activity by binding to sterols in the fungal cell membrane. The drug is not active against organisms (e.g., bacteria) that do not contain sterols in their cell membrane. As a result of this binding, the membrane is no longer able to function as a selective barrier, and potassium and other cellular constituents are lost., ... /Antimicrobial/ agents that act directly on the cell membrane of the microorganism, affecting permeability and leading to leakage of intracellular compounds; these include ... the polyene antifungal agents nystatin ... which bind to cell-wall sterols ... | |
| Record name | NYSTATIN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3138 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Light yellow powder, Yellow to tan powder | |
CAS No. |
34786-70-4, 1400-61-9 | |
| Record name | Nystatin A1 | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=34786-70-4 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Nystatin A1 | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0034786704 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | (7R,10R)-8,9-Dideoxy-28,29-dihydro-7,10-dihydroxyamphotericin B | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID80872323 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Nystatin | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.014.317 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | NYSTATIN A1 | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/W1LX4T91WI | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | NYSTATIN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3138 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Melting Point |
Gradually decomp above 160 °C without melting by 250 °C | |
| Record name | NYSTATIN | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3138 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.















