Moxifloxacine
Vue d'ensemble
Description
La moxifloxacine est un antibiotique de la famille des fluoroquinolones synthétiques utilisé pour traiter diverses infections bactériennes. Elle est efficace contre les bactéries à Gram positif et à Gram négatif. La this compound est généralement utilisée pour traiter les infections des voies respiratoires, les infections cutanées et les infections intra-abdominales. Elle est disponible sous forme orale, intraveineuse et ophtalmique .
Mécanisme D'action
La moxifloxacine exerce ses effets antibactériens en inhibant les enzymes topoisomérase II (ADN gyrase) et topoisomérase IV. Ces enzymes sont essentielles pour la réplication, la transcription et la réparation de l’ADN bactérien. En inhibant ces enzymes, la this compound empêche les bactéries de répliquer et de réparer leur ADN, ce qui conduit à la mort des cellules bactériennes .
Applications De Recherche Scientifique
FDA-Approved Indications
Moxifloxacin is primarily indicated for the treatment of:
- Respiratory Tract Infections : Effective against community-acquired pneumonia (CAP) caused by Streptococcus pneumoniae and Mycoplasma pneumoniae.
- Sinusitis : Approved for acute bacterial sinusitis.
- Chronic Bronchitis : Used for acute bacterial exacerbations.
- Skin and Skin Structure Infections (SSSIs) : Effective against complicated and uncomplicated SSSIs, including those caused by Staphylococcus aureus and Streptococcus pyogenes.
- Intra-abdominal Infections : Approved for complicated intra-abdominal infections.
- Plague : Treatment of pneumonic and septicemic forms caused by Yersinia pestis.
Moxifloxacin's broad-spectrum activity makes it a valuable option for treating various infections, especially when resistance to other antibiotics is a concern .
Off-Label Uses
In addition to its approved indications, moxifloxacin has several off-label applications:
- Urinary Tract Infections (UTIs) : Sometimes used when first-line treatments are ineffective.
- Chronic Bacterial Prostatitis : Employed after the failure of other antibiotics.
- Multidrug-Resistant Tuberculosis (MDR-TB) : Included in treatment regimens for MDR-TB, supported by guidelines from major health organizations such as the American Thoracic Society and the CDC .
- Exacerbations of Chronic Obstructive Pulmonary Disease (COPD) : Utilized when there is a risk of Pseudomonas aeruginosa infection.
Treatment of Respiratory Infections
A study comparing moxifloxacin to amoxicillin demonstrated a clinical success rate of 94.1% for moxifloxacin versus 91.7% for amoxicillin in treating respiratory infections. This indicates that moxifloxacin is highly effective and may be preferred in cases where patients have a history of antibiotic resistance .
Skin and Skin Structure Infections
Moxifloxacin has shown comparable efficacy to beta-lactam antibiotics in treating SSSIs. In randomized controlled trials, it was found to be as effective as cephalexin for uncomplicated SSSIs, with bacteriological success rates supporting its use in complicated cases .
Tuberculosis Treatment
Moxifloxacin's role in treating MDR-TB has been extensively studied. It is often included in combination therapy regimens aimed at shortening treatment duration. However, optimal dosing and long-term safety data remain under investigation .
Ocular Applications
A case report highlighted severe corneal toxicity following the use of topical moxifloxacin, indicating potential adverse effects that warrant further investigation into its safety profile .
Efficacy Summary Table
| Indication | Clinical Success Rate | Notes |
|---|---|---|
| Community-Acquired Pneumonia | 94.1% | Compared favorably to amoxicillin |
| Skin and Skin Structure Infections | 88.2% | Comparable efficacy to cephalexin |
| Multidrug-Resistant Tuberculosis | Not established | Used in combination therapy; ongoing studies needed |
| Ocular Toxicity | N/A | Case report indicates potential severe adverse effects |
Analyse Biochimique
Biochemical Properties
Moxifloxacin’s role in biochemical reactions primarily involves the inhibition of bacterial enzymes. It inhibits the enzymes topoisomerase II (DNA gyrase) and topoisomerase IV, which are essential for bacterial DNA replication and transcription . By inhibiting these enzymes, moxifloxacin prevents bacteria from duplicating their DNA, thereby stopping their growth and proliferation .
Cellular Effects
Moxifloxacin has been shown to have significant effects on various types of cells and cellular processes. For instance, it has been found to inhibit the yeast to Hyphal morphogenesis by affecting signaling pathways . It also arrested the cell cycle of Candida albicans at the S phase . Moreover, it has been observed to have photostability and reduced phototoxicity on melanocytes .
Molecular Mechanism
The molecular mechanism of action of moxifloxacin involves its interaction with bacterial enzymes. It binds to and inhibits the activity of DNA gyrase and topoisomerase IV . These enzymes are responsible for maintaining the superhelical structure of DNA and are required for DNA replication and transcription, DNA repair, recombination, and transposition . By inhibiting these enzymes, moxifloxacin prevents the bacteria from duplicating their DNA, leading to their death .
Temporal Effects in Laboratory Settings
In laboratory settings, moxifloxacin has shown a dose-dependent reduction in bacterial density over time . It has been found to be the most effective among the four fluoroquinolones tested against penicillin-resistant streptococcal pneumonia in a mouse model . Moreover, it has been observed that moxifloxacin has a good stability profile, with no significant degradation over time .
Dosage Effects in Animal Models
In animal models, the effects of moxifloxacin have been observed to vary with different dosages. In rabbit models of meningitis, treatment with moxifloxacin resulted in a dose-dependent reduction in bacterial density . In a murine model of disseminated Mycobacterium tuberculosis infection, moxifloxacin was equivalent in its ability to prevent mortality and reduce the concentration of bacteria in spleens to other antibiotics tested .
Metabolic Pathways
Moxifloxacin undergoes Phase II metabolism in humans, resulting in the formation of two main metabolites: N-sulphate (M1) and acylglucuronide (M2) . These metabolites are pharmacologically inactive . Moxifloxacin is eliminated via metabolic, renal, and biliary/faecal routes .
Transport and Distribution
Moxifloxacin is rapidly absorbed in humans, with a high bioavailability of approximately 90% . It has a good distribution to saliva, interstitial fluids, and lung tissues . About 22% of a moxifloxacin dose is excreted via the kidneys as unmetabolised moxifloxacin .
Méthodes De Préparation
Voies de synthèse et conditions réactionnelles : La moxifloxacine est synthétisée par un processus en plusieurs étapes. Les étapes clés comprennent la formation du noyau quinoléine, suivie de l’introduction du groupe cyclopropyle, du groupe méthoxy et de la portion diazabicyclononane. La synthèse implique généralement les étapes suivantes :
- Formation du noyau quinoléine par une réaction de condensation entre un dérivé d’aniline et un β-cétoester.
- Introduction du groupe cyclopropyle par une réaction de cyclopropanation.
- Addition du groupe méthoxy par une réaction de substitution nucléophile.
- Formation de la portion diazabicyclononane par une réaction de cyclisation .
Méthodes de production industrielle : La production industrielle de this compound implique une synthèse à grande échelle utilisant des conditions réactionnelles optimisées pour garantir un rendement élevé et une pureté optimale. Le processus comprend :
- L’utilisation de matières premières et de réactifs de haute pureté.
- L’optimisation des conditions réactionnelles telles que la température, la pression et le pH.
- La mise en œuvre de techniques de purification telles que la cristallisation et la chromatographie pour obtenir le produit final .
Analyse Des Réactions Chimiques
Types de réactions : La moxifloxacine subit diverses réactions chimiques, notamment :
Oxydation : La this compound peut être oxydée pour former des N-oxydes de quinoléine.
Réduction : La réduction de la this compound peut conduire à la formation de dérivés dihydroquinoléine.
Substitution : Des réactions de substitution nucléophile peuvent se produire au niveau du groupe méthoxy ou de la portion diazabicyclononane
Réactifs et conditions courants :
Oxydation : Les agents oxydants courants comprennent le peroxyde d’hydrogène et le permanganate de potassium.
Réduction : Des agents réducteurs tels que le borohydrure de sodium et l’hydrure de lithium et d’aluminium sont utilisés.
Substitution : Des nucléophiles tels que les amines et les thiols sont utilisés dans les réactions de substitution
Produits majeurs :
Oxydation : N-oxydes de quinoléine.
Réduction : Dérivés dihydroquinoléine.
Substitution : Dérivés de quinoléine substitués par des groupes amino ou thio
Comparaison Avec Des Composés Similaires
La moxifloxacine est comparée à d’autres antibiotiques de la famille des fluoroquinolones tels que la ciprofloxacine et la lévofloxacine :
Ciprofloxacine : La this compound a un spectre d’activité plus large et est plus efficace contre les bactéries à Gram positif que la ciprofloxacine.
Lévofloxacine : La this compound a une demi-vie plus longue et une meilleure pénétration tissulaire, ce qui la rend plus efficace pour certains types d’infections.
Composés similaires :
- Ciprofloxacine
- Lévofloxacine
- Ofloxacine
- Norfloxacine
Les propriétés uniques de la this compound, telles que son large spectre d’activité et son excellente pénétration tissulaire, en font un antibiotique précieux dans le traitement de diverses infections bactériennes.
Activité Biologique
Moxifloxacin is a broad-spectrum fluoroquinolone antibiotic known for its potent bactericidal activity against various bacterial pathogens. Its biological activity is primarily attributed to its mechanism of action, pharmacokinetics, and clinical efficacy in treating infections. This article delves into the biological activity of moxifloxacin, supported by data tables, case studies, and detailed research findings.
Moxifloxacin exerts its antibacterial effects through the inhibition of bacterial DNA synthesis. It specifically targets two essential enzymes involved in DNA replication: DNA gyrase and topoisomerase IV . By binding to these enzymes, moxifloxacin prevents the unwinding and repair of DNA strands, leading to bacterial cell death. This mechanism is crucial for its effectiveness against a wide range of pathogens, including both Gram-positive and Gram-negative bacteria .
Pharmacokinetics
Moxifloxacin has favorable pharmacokinetic properties, which enhance its clinical efficacy:
- Absorption : Rapidly absorbed with a bioavailability exceeding 90%.
- Distribution : Widely distributed in body tissues and fluids, with good penetration into respiratory tissues and cerebrospinal fluid.
- Metabolism : Primarily metabolized in the liver, with minimal renal excretion.
- Half-life : Approximately 10-12 hours, allowing for once-daily dosing.
These properties contribute to its effectiveness in treating various infections, including respiratory tract infections and complicated intra-abdominal infections (cIAIs) .
Case Studies and Clinical Trials
-
Community-Acquired Pneumonia (CAP) :
- Study : The MOTIV study evaluated moxifloxacin monotherapy in hospitalized patients with CAP.
- Results : Clinical cure rates were 86.9% for moxifloxacin compared to 89.9% for the comparator regimen (95% CI: -8.1% to 2.2%). Bacteriological success rates were 83.3% for moxifloxacin versus 85.1% for the comparator .
- Complicated Intra-Abdominal Infections (cIAIs) :
- Tuberculosis Treatment :
Efficacy Against Biofilms
Recent studies have highlighted moxifloxacin's activity against biofilms formed by clinical isolates. While it exhibits strong bactericidal activity, its effectiveness can be reduced in biofilm environments due to lower local concentrations .
Comparative Efficacy
A comparison between moxifloxacin and amoxicillin revealed that moxifloxacin had a higher clinical success rate (94.1% vs. 91.7%) at treatment completion .
Summary of Key Findings
| Study Type | Pathogen | Moxifloxacin Success Rate | Comparator Success Rate | Notes |
|---|---|---|---|---|
| MOTIV Study | CAP | 86.9% | 89.9% | No significant differences in adverse events |
| PROMISE Study | cIAIs | Non-inferior | Ertapenem | Effective over 21-28 days |
| Tuberculosis Pilot Study | Mycobacterium tuberculosis | Significant activity | Ethambutol | Potential for shorter treatment duration |
| Moxifloxacin vs Amoxicillin | Various | 94.1% | 91.7% | Higher success rate in clinical outcomes |
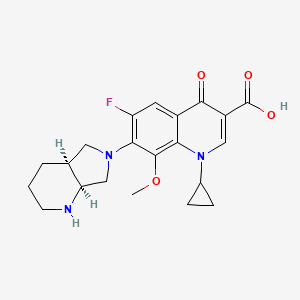
Propriétés
IUPAC Name |
7-[(4aS,7aS)-1,2,3,4,4a,5,7,7a-octahydropyrrolo[3,4-b]pyridin-6-yl]-1-cyclopropyl-6-fluoro-8-methoxy-4-oxoquinoline-3-carboxylic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C21H24FN3O4/c1-29-20-17-13(19(26)14(21(27)28)9-25(17)12-4-5-12)7-15(22)18(20)24-8-11-3-2-6-23-16(11)10-24/h7,9,11-12,16,23H,2-6,8,10H2,1H3,(H,27,28)/t11-,16+/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
FABPRXSRWADJSP-MEDUHNTESA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
COC1=C2C(=CC(=C1N3CC4CCCNC4C3)F)C(=O)C(=CN2C5CC5)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
COC1=C2C(=CC(=C1N3C[C@@H]4CCCN[C@@H]4C3)F)C(=O)C(=CN2C5CC5)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C21H24FN3O4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID3048491 | |
| Record name | Moxifloxacin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3048491 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
401.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Moxifloxacin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014363 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
1.68e-01 g/L | |
| Record name | Moxifloxacin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014363 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
The bactericidal action of moxifloxacin results from inhibition of the enzymes topoisomerase II (DNA gyrase) and topoisomerase IV. DNA gyrase is an essential enzyme that is involved in the replication, transcription and repair of bacterial DNA. Topoisomerase IV is an enzyme known to play a key role in the partitioning of the chromosomal DNA during bacterial cell division., The fluoroquinolone antibiotic moxifloxacin has been associated with the acquired long QT syndrome and is used as a positive control in the evaluation of the QT-interval prolonging potential of new drugs. In common with other QT-prolonging agents, moxifloxacin is known to inhibit the hERG potassium K+ channel, but at present there is little mechanistic information available on this action. This study was conducted in order to characterise the inhibition of hERG current (I(hERG)) by moxifloxacin, and to determine the role in drug binding of the S6 aromatic amino-acid residues Tyr652 and Phe656. hERG currents were studied using whole-cell patch clamp (at room temperature and at 35-37 degrees C) in an HEK293 cell line stably expressing hERG channels. Moxifloxacin reversibly inhibited currents in a dose-dependent manner. We investigated the effects of different voltage commands to elicit hERG currents on moxifloxacin potency. Using a 'step-ramp' protocol, the IC50 was 65 uM at room temperature and 29 microM at 35 degrees C. When a ventricular action potential waveform was used to elicit currents, the IC50 was 114 microM. Block of hERG by moxifloxacin was found to be voltage-dependent, occurred rapidly and was independent of stimulation frequency. Mutagenesis of the S6 helix residue Phe656 to Ala failed to eliminate or reduce the moxifloxacin-mediated block whereas mutation of Tyr652 to Ala reduced moxifloxacin block by approximately 66%. Our data demonstrate that moxifloxacin blocks the hERG channel with a preference for the activated channel state. The Tyr652 but not Phe656 S6 residue is involved in moxifloxacin block of hERG, concordant with an interaction in the channel inner cavity., The bactericidal action of moxifloxacin results from inhibition of the topoisomerase II (DNA gyrase) and topoisomerase IV required for bacterial DNA replication, transcription, repair, and recombination. It appears that the C8-methoxy moiety contributes to enhanced activity and lower selection of resistant mutants of Gram-positive bacteria compared to the C8-H moiety. The presence of the bulky bicycloamine substituent at the C-7 position prevents active efflux, associated with the NorA or pmrA genes seen in certain Gram-positive bacteria., Torsade de pointes (TdP) is increasingly recognized as a complication of drug therapy. The most common cause of drug-induced QT prolongation is inhibition of the rapidly activating component of the delayed potassium current (I(Kr)). Moxifloxacin, a widely used fluoroquinolone, is a weak I(Kr) inhibitor and has been associated with QT prolongation., Fluoroquinolones prolong the QT interval by blocking voltage-gated potassium channels, especially the rapid component of the delayed rectifier potassium current I(Kr), expressed by HERG (the human ether-a-go-go-related gene). According to the available case reports and clinical studies, moxifloxacin carries the greatest risk of QT prolongation from all available quinolones in clinical practice and it should be used with caution in patients with predisposing factors for Torsades de pointes (TdP). | |
| Record name | Moxifloxacin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00218 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Moxifloxacin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8026 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
CAS No. |
151096-09-2, 354812-41-2 | |
| Record name | Moxifloxacin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=151096-09-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Moxifloxacin [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0151096092 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Moxifloxacin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00218 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Moxifloxacin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3048491 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 3-Quinolinecarboxylic acid, 1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.129.459 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | MOXIFLOXACIN | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/U188XYD42P | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Moxifloxacin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8026 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Moxifloxacin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014363 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
238-242 °C, 208-209 °C (decomposes), 238 - 242 °C | |
| Record name | Moxifloxacin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00218 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Moxifloxacin | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/8026 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Moxifloxacin | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014363 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.















