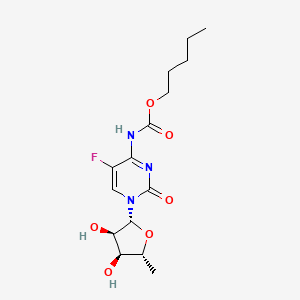
Capecitabine
Vue d'ensemble
Description
La capecitabine est un agent chimiothérapeutique administré par voie orale utilisé dans le traitement de divers cancers, notamment les cancers métastatiques du sein et du côlon . Il s'agit d'un prod médicament qui est converti enzymatiquement en fluorouracile dans la tumeur, où il inhibe la synthèse de l'ADN et ralentit la croissance des tissus tumoraux . La this compound appartient à un groupe d'agents antinéoplasiques appelés antimetabolites, qui tuent les cellules cancéreuses en interférant avec la synthèse de l'ADN .
Méthodes De Préparation
Voies de synthèse et conditions réactionnelles : La capecitabine est synthétisée par un processus en plusieurs étapes. La synthèse implique la réaction de la 5-désoxy-5-fluorocytidine avec le chloroformiate de pentyle pour former le composé intermédiaire, qui est ensuite réagi davantage pour produire la this compound . Les conditions réactionnelles impliquent généralement l'utilisation de solvants comme l'alcool éthylique et nécessitent un contrôle précis de la température et du pH pour assurer l'obtention du produit souhaité .
Méthodes de production industrielle : La production industrielle de this compound implique une synthèse à grande échelle utilisant des conditions réactionnelles similaires à celles de la synthèse en laboratoire. Le processus comprend le tamisage, le pelletage, le séchage et le façonnage du composé pour assurer la stabilité et la qualité du produit . Des techniques de pointe comme la chromatographie liquide haute performance (HPLC) et la chromatographie sur couche mince haute performance (HPTLC) sont utilisées pour le contrôle qualité et la surveillance de la concentration plasmatique de la this compound .
Analyse Des Réactions Chimiques
Metabolic Pathway of Capecitabine
This compound’s metabolism involves a three-step enzymatic cascade, converting the prodrug into its active metabolite, 5-fluorouracil (5-FU) .
Step 1: Carboxylesterase Hydrolysis
-
Reaction: this compound → 5′-Deoxy-5-fluorocytidine (5′-DFCR)
-
Mechanism: CES1 cleaves the pentyl ester group, initiating activation .
Step 2: Cytidine Deaminase Conversion
-
Reaction: 5′-DFCR → 5′-Deoxy-5-fluorouridine (5′-DFUR)
-
Enzyme: Cytidine deaminase (CDA), widespread in tissues and tumors .
-
Mechanism: Deamination removes the cytidine base, forming a uridine analog .
Step 3: Thymidine Phosphorylase Activation
-
Reaction: 5′-DFUR → 5-FU
-
Enzyme: Thymidine phosphorylase (TP), overexpressed in tumors .
-
Mechanism: TP cleaves the sugar-phosphate bond, releasing 5-FU preferentially in tumor tissue .
Metabolites of 5-FU
Key Mechanisms
-
Thymidylate Synthase Inhibition
-
RNA Toxicity
Metabolic Degradation
-
Dihydropyrimidine Dehydrogenase (DPD)
-
Dihydropyrimidinase
-
β-Ureidopropionase
Pharmacokinetic Data
| Parameter | Value |
|---|---|
| Half-life | ~0.75 hours |
| Clearance | 775 ± 213 mL/min |
| Urinary Excretion | 96% (3% unchanged) |
Hand-Foot Syndrome (HFS)
-
Mechanism: Elevated thymidine phosphorylase (TP) in palmoplantar tissues converts 5′-DFUR to 5-FU locally, causing pyroptosis via gasdermin E (GSDME) .
-
Key Findings:
Enzymatic Polymorphisms
-
CES1 Variants: Influence this compound bioactivation, with reduced CES1 activity linked to altered plasma metabolite levels .
-
DPD Deficiency: Increases 5-FU toxicity due to impaired degradation .
Cytochrome P450 Interactions
Applications De Recherche Scientifique
Clinical Applications
1. Colorectal Cancer Treatment
- Adjuvant Therapy : Capecitabine has been approved for adjuvant treatment in patients with stage III colon cancer following surgery. Studies demonstrate its non-inferiority to traditional intravenous 5-fluorouracil (5-FU) regimens, with comparable overall survival rates .
- Metastatic Colorectal Cancer : In first-line treatment settings for metastatic colorectal cancer, this compound has shown superior response rates compared to intravenous regimens. A significant study reported an overall response rate of 26% for this compound versus 17% for 5-FU plus leucovorin .
2. Off-Label Uses
- This compound is increasingly used off-label for various malignancies including breast cancer and gastric cancer. Its efficacy in combination with other chemotherapeutic agents such as oxaliplatin has been explored in clinical trials, showing promising results .
Efficacy and Safety Profile
The safety profile of this compound is generally favorable compared to traditional chemotherapy agents. Common side effects include hand-foot syndrome, diarrhea, and stomatitis; however, it has demonstrated lower incidences of severe neutropenia and alopecia compared to intravenous therapies .
Comparative Efficacy Table
| Treatment Regimen | Overall Response Rate | Median Time to Progression | Median Overall Survival |
|---|---|---|---|
| This compound | 26% | 4.6 months | 12.9 months |
| Intravenous 5-FU + Leucovorin | 17% | 4.7 months | 12.8 months |
Case Studies
Case Study 1: Metastatic Colorectal Cancer
A phase III trial enrolled over 1200 patients with untreated metastatic colorectal cancer, comparing this compound with standard intravenous therapies. Results indicated that this compound not only matched the efficacy of traditional treatments but also improved patient quality of life due to its oral administration route .
Case Study 2: Elderly Patients
A study focusing on elderly patients demonstrated that this compound was well tolerated even among those with comorbid conditions. The median overall survival was reported at approximately 11 months, showcasing its applicability in older demographics who may struggle with intravenous therapies .
Mécanisme D'action
Capecitabine is metabolized to fluorouracil in vivo by carboxylesterases, cytidine deaminase, and thymidine phosphorylase/uridine phosphorylase sequentially . Fluorouracil is further metabolized into three main active metabolites: 5-fluorouridine triphosphate (5-FUTP), 5-fluoro-2’-deoxyuridine-5’-triphosphate (5-FdUTP), and 5-fluoro-2’-deoxyuridine-5’-monophosphate (5-FdUMP) . These metabolites inhibit DNA synthesis by incorporating into RNA and DNA, leading to cell death . The molecular targets include thymidylate synthase, which is inhibited by 5-FdUMP, and RNA polymerase, which is inhibited by 5-FUTP .
Comparaison Avec Des Composés Similaires
Comparée au fluorouracile, la capecitabine a l'avantage d'être administrée par voie orale, ce qui améliore la compliance des patients . Le tégafur est un autre prod médicament du fluorouracile, mais il est souvent associé à des toxicités neurologiques . La conversion enzymatique unique de la this compound en fluorouracile dans les tissus tumoraux en fait une option de traitement plus ciblée et plus efficace .
Composés similaires :
- Fluorouracile
- Tégafur
- Ibrance (palbociclib)
- Enhertu (fam-trastuzumab deruxtecan)
Les propriétés uniques et l'action ciblée de la this compound en font un composé précieux dans le traitement du cancer et la recherche scientifique.
Activité Biologique
Capecitabine is an oral prodrug that is primarily used in the treatment of various cancers, particularly breast and colorectal cancers. Its biological activity is closely linked to its metabolism into 5-fluorouracil (5-FU), which exerts its cytotoxic effects through multiple mechanisms. This article reviews the biological activity of this compound, including its mechanism of action, clinical efficacy, and safety profile, supported by data tables and relevant case studies.
This compound is converted to 5-FU in the body through a series of enzymatic reactions. The key enzymes involved in this conversion include carboxylesterases, cytidine deaminase, and thymidine phosphorylase (TP). The active metabolites of 5-FU inhibit thymidylate synthase, disrupt DNA and RNA synthesis, and induce apoptosis in cancer cells .
Key Metabolites and Their Functions:
- 5-Fluoro-2’-deoxyuridine monophosphate (FdUMP) : Inhibits thymidylate synthase.
- 5-Fluorouridine triphosphate (FUTP) : Incorporates into RNA, disrupting protein synthesis.
- 5-Fluorodeoxyuridine triphosphate (FdUTP) : Interferes with DNA synthesis.
Clinical Efficacy
This compound has been evaluated in numerous clinical trials for its efficacy in treating various types of cancer.
Case Studies and Clinical Trials
- Adjuvant Therapy in Breast Cancer : A Phase III trial involving 876 patients assessed the efficacy of this compound after standard chemotherapy in early triple-negative breast cancer. The results indicated no significant difference in disease-free survival (DFS) compared to observation, but non-basal phenotype patients showed improved outcomes with this compound .
- Gastric Cancer Treatment : In a study comparing this compound plus cisplatin to S-1 plus cisplatin for advanced gastric cancer, this compound demonstrated a median overall survival (OS) of 14.2–17.7 months with an overall response rate of 49.2%–58.5% .
- Maintenance Therapy : A study on maintenance therapy with this compound following induction chemotherapy showed promising results in prolonging survival without significant toxicity .
Safety Profile
The safety profile of this compound includes various adverse effects, with hand-foot syndrome being one of the most common. In clinical trials, approximately 40.6% of patients experienced grade 3 or greater adverse events when treated with this compound compared to 15.5% in observation groups .
Adverse Events Reported:
- Grade 3 or Greater AEs : Neutropenia, anemia, nausea.
- Serious Adverse Events (SAEs) : Reported in 5.3% of patients receiving this compound.
Pharmacodynamics and Pharmacokinetics
This compound's pharmacodynamics are influenced by its selective conversion to 5-FU in tumor tissues where TP is expressed. This selective activation minimizes systemic toxicity while maximizing local cytotoxic effects on tumors .
Data Summary Table
Q & A
Basic Research Questions
Q. What are the key considerations when designing a non-inferiority trial to compare capecitabine with fluorouracil in advanced gastrointestinal cancers?
- Methodological Answer : Non-inferiority trials require rigorous margin selection based on historical efficacy data and clinical relevance. For example, in the Cunningham et al. (2008) trial, the predefined non-inferiority margin for hazard ratio (HR) was set at 1.23, ensuring this compound's equivalence to fluorouracil in overall survival (OS) . Researchers should justify margins using prior meta-analyses, ensure adequate power, and address potential confounding variables (e.g., dosing schedules, patient stratification). Blinding and randomization protocols are critical to minimize bias .
Q. How do toxicity profiles of this compound-based regimens influence patient selection in clinical trials?
- Methodological Answer : Toxicity data (e.g., grade 3/4 diarrhea, hand-foot syndrome) should guide inclusion/exclusion criteria. For instance, oxaliplatin-capecitabine combinations show lower renal toxicity and alopecia compared to cisplatin-fluorouracil but may require dose adjustments for neuropathy . Use validated tools like CTCAE (Common Terminology Criteria for Adverse Events) to standardize reporting and stratify patients by comorbidities (e.g., renal impairment) .
Q. What statistical methods are appropriate for analyzing survival outcomes in this compound trials?
- Methodological Answer : Kaplan-Meier curves with log-rank tests and Cox proportional hazards models (reporting HRs and 95% confidence intervals) are standard for OS and progression-free survival (PFS). For example, Cunningham et al. (2008) used Cox regression to demonstrate non-inferiority (HR=0.86 for this compound vs. fluorouracil) . Pre-specify subgroup analyses to explore heterogeneity (e.g., by tumor stage or biomarker status) .
Advanced Research Questions
Q. How can researchers reconcile contradictory efficacy results of this compound in pancreatic cancer across phase III trials?
- Methodological Answer : Contradictions (e.g., OS trends in Cunningham et al. (2009) vs. meta-analyses) require sensitivity analyses and evaluation of trial design differences. For instance, the GEM-CAP trial (HR=0.86, P=0.08) showed marginal OS benefit, while pooled meta-analyses (HR=0.86, P=0.02) confirmed significance . Investigate heterogeneity via meta-regression (e.g., patient demographics, treatment adherence) and assess publication bias using funnel plots .
Q. What strategies validate predictive biomarkers (e.g., TYMP, immune signatures) for this compound benefit in triple-negative breast cancer (TNBC)?
- Methodological Answer : Use hypothesis-driven approaches with prespecified endpoints. Asleh et al. (2020) applied a 770-gene panel and custom this compound-metabolism genes, followed by Cox models with interaction tests (e.g., P-interaction=0.01 for cytotoxic cells). Adjust for multiplicity (e.g., Bonferroni correction) and validate findings in independent cohorts. Functional assays (e.g., TYMP enzyme activity) strengthen mechanistic plausibility .
Q. How should meta-analyses address heterogeneity when comparing this compound/5-FU combinations in colorectal cancer?
- Methodological Answer : Employ random-effects models to account for between-study variability. Wang et al. (2012) used RevMan 4.2 to pool 6 trials (n=2,189), reporting I² statistics to quantify heterogeneity. Stratify by protocol differences (e.g., oxaliplatin dosing) and perform subgroup analyses (e.g., RAS mutation status) to identify effect modifiers .
Q. Methodological Recommendations
- For Experimental Design : Use PICO framework (Population: e.g., metastatic CRC; Intervention: this compound; Comparison: 5-FU; Outcome: OS) to structure research questions .
- For Data Contradictions : Apply Bradford Hill criteria (e.g., consistency, biological gradient) to assess causality in conflicting results .
- For Biomarker Studies : Follow REMARK guidelines for transparent reporting and include pre-analytical variables (e.g., tissue fixation methods) .
Propriétés
IUPAC Name |
pentyl N-[1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-methyloxolan-2-yl]-5-fluoro-2-oxopyrimidin-4-yl]carbamate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C15H22FN3O6/c1-3-4-5-6-24-15(23)18-12-9(16)7-19(14(22)17-12)13-11(21)10(20)8(2)25-13/h7-8,10-11,13,20-21H,3-6H2,1-2H3,(H,17,18,22,23)/t8-,10-,11-,13-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
GAGWJHPBXLXJQN-UORFTKCHSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCCCCOC(=O)NC1=NC(=O)N(C=C1F)C2C(C(C(O2)C)O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CCCCCOC(=O)NC1=NC(=O)N(C=C1F)[C@H]2[C@@H]([C@@H]([C@H](O2)C)O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C15H22FN3O6 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID3046451 | |
| Record name | Capecitabine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3046451 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
359.35 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Capecitabine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015233 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
In water, 26 mg/mL at 20 °C, 2.48e-01 g/L | |
| Record name | Capecitabine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01101 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | CAPECITABINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7656 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Capecitabine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015233 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Capecitabine is a prodrug that is selectively tumour-activated to its cytotoxic moiety, fluorouracil, by thymidine phosphorylase, an enzyme found in higher concentrations in many tumors compared to normal tissues or plasma. Fluorouracil is further metabolized to two active metabolites, 5-fluoro-2'-deoxyuridine 5'-monophosphate (FdUMP) and 5-fluorouridine triphosphate (FUTP), within normal and tumour cells. These metabolites cause cell injury by two different mechanisms. First, FdUMP and the folate cofactor, N5-10-methylenetetrahydrofolate, bind to thymidylate synthase (TS) to form a covalently bound ternary complex. This binding inhibits the formation of thymidylate from 2'-deaxyuridylate. Thymidylate is the necessary precursor of thymidine triphosphate, which is essential for the synthesis of DNA, therefore a deficiency of this compound can inhibit cell division. Secondly, nuclear transcriptional enzymes can mistakenly incorporate FUTP in place of uridine triphosphate (UTP) during the synthesis of RNA. This metabolic error can interfere with RNA processing and protein synthesis through the production of fraudulent RNA., Capecitabine is a prodrug and has little pharmacologic activity until it is converted to fluorouracil, an antimetabolite. Because capecitabine is converted to fluorouracil by enzymes that are expressed at higher concentrations in many tumors than in adjacent normal tissues or plasma, it is thought that high tumor concentrations of the active drug may be achieved with less systemic toxicity. Fluorouracil is metabolized in both normal and tumor cells to 5-fluoro-2'-deoxyuridine 5'-monophosphate (FdUMP) and 5-fluorouridine triphosphate (FUTP). Although the precise mechanisms of action of fluorouracil have not been fully elucidated, the main mechanism is thought to be the binding of the deoxyribonucleotide of the drug (FdUMP) and the folate cofactor (N5-10-methylenetetrahydrofolate) to thymidylate synthase (TS) to form a covalently bound ternary complex, which inhibits the formation of thymidylate from 2'-deoxyuridylate, thereby interfering with DNA synthesis. In addition, FUTP can be incorporated into RNA in place of uridine triphosphate (UTP), producing a fraudulent RNA and interfering with RNA processing and protein synthesis. Capecitabine has been shown to be active in xenograft tumors that are resistant to fluorouracil indicating incomplete cross-resistance between the drugs., In this report, /the authors/ investigated whether apoptosis induced by capecitabine was mediated by the Fas/FasL system. To achieve this goal, a specific in vitro coculture model mixing hepatoma and human colorectal cell line was used. A bystander effect was observed between HepG2 and LS174T cells treated with capecitabine. Besides this, Xeloda showed a 7-fold higher cytotoxicity and markedly stronger apoptotic potential in thymidine phosphorylase (TP)-transfected LS174T-c2 cells. The striking enhancement of thymidylate synthase inhibition that we observed in cells with high TP activity was most probably at the origin of the potentiation of capecitabine antiproliferative efficacy. In addition, this increase of sensitivity was accompanied by a strong overexpression of the CD95-Fas receptor on the cell surface. Both Fas and FasL mRNA expression were triggered after exposing TP+ cells to the drug. This implication of Fas in Xeloda-induced apoptosis was next confirmed by using antagonistic anti-Fas and anti-FasL antibodies that proved to reverse capecitabine antiproliferative activity, thus highlighting the key role that Fas could play in the optimization of an antitumor response to fluoropyrimidine drugs. /The/ data, therefore, show that TP plays a key role in the capecitabine activity and that the Fas/FasL system could be considered as a new determinant for Xeloda efficacy. | |
| Record name | Capecitabine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01101 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | CAPECITABINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7656 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Impurities |
2',3'-di-O-acetyl-5'-deoxy-5-fluorocytidine; 5'-deoxy-5-fluoro-N4-(2-methyl-1-butyloxycarbonyl)cytidine; 5'-deoxy-5-fluoro-N4-(3-methyl-1-butyloxycarbonyl)cytidine; [1-[5-deoxy-3-O-(5-deoxy-beta-D-ribofuranosyl)-beta-D-ribofuranosyl]-5-fluoro-2-oxo-1,2-dihydropyrimidin-4-yl]-carbamic acid pentyl ester; [1-[5-deoxy-2-O-(5-deoxy-beta-D-ribofuranosyl)-beta-D-ribofuranosyl]-5-fluoro-2-oxo-1,2-dihydropyrimidin-4-yl]-carbamic acid pentyl ester; [1-[5-deoxy-3-O-(5-deoxy-alpha-D-ribofuranosyl)-beta-D-ribofuranosyl]-5-fluoro-2-oxo-1,2-dihydropyrimidin-4-yl]-carbamic acid pentyl ester; 2',3'-di-O-acetyl-5'-deoxy-5-fluoro-N4-(pentyloxycarbonyl)cytidine | |
| Record name | CAPECITABINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7656 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
White to off-white crystalline powder, Crystals from ethyl acetate | |
CAS No. |
154361-50-9 | |
| Record name | Capecitabine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=154361-50-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Capecitabine [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0154361509 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Capecitabine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01101 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Capecitabine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3046451 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Cytidine, 5'-deoxy-5-fluoro-N-[(pentyloxy)carbonyl] | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.112.980 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CAPECITABINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/6804DJ8Z9U | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | CAPECITABINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7656 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Capecitabine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015233 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
110-121 °C, 110 - 121 °C | |
| Record name | Capecitabine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB01101 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | CAPECITABINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7656 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Capecitabine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0015233 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.















