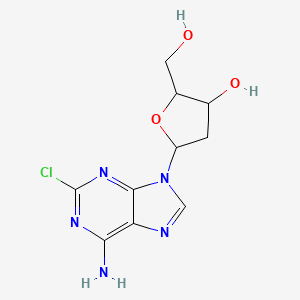
Cladribine
Vue d'ensemble
Description
La cladribine, également connue sous le nom de 2-chlorodésoxyadénosine, est un analogue synthétique de la nucléoside purine. Elle est principalement utilisée dans le traitement de la leucémie à tricholeucocytes et de la sclérose en plaques. La this compound imite la nucléoside désoxyadénosine mais est résistante à la dégradation par l’enzyme adénosine désaminase, ce qui lui permet de s’accumuler dans les cellules ciblées et d’interférer avec la synthèse de l’ADN .
Mécanisme D'action
La cladribine exerce ses effets en imitant la désoxyadénosine et en étant incorporée dans l’ADN. Une fois à l’intérieur de la cellule, elle est phosphorylée par la désoxycytidine kinase pour produire du 2-chlorodésoxyadénosine triphosphate. Cette forme active perturbe la synthèse et la réparation de l’ADN, conduisant à l’apoptose. La this compound cible sélectivement les lymphocytes en raison du rapport kinase : phosphatase élevé dans ces cellules, ce qui la rend efficace dans le traitement des maladies impliquant une activité lymphocytaire anormale .
Composés Similaires :
Pentostatine : Un autre analogue de la purine utilisé pour traiter la leucémie à tricholeucocytes.
Fludarabine : Un analogue de la purine utilisé dans le traitement de la leucémie lymphoïde chronique.
Unicité de la this compound : La résistance de la this compound à l’adénosine désaminase et son ciblage sélectif des lymphocytes la rendent unique parmi les analogues de la purine. Sa capacité à être administrée par voie orale pour le traitement de la sclérose en plaques la distingue également des autres composés similaires .
Applications De Recherche Scientifique
Cladribine has a wide range of scientific research applications:
Chemistry: It is used as a model compound to study nucleoside analogs and their interactions with enzymes.
Biology: this compound is utilized to investigate the mechanisms of lymphocyte depletion and immune modulation.
Medicine: It is a critical drug in the treatment of hairy cell leukemia and multiple sclerosis. .
Industry: this compound’s synthesis and production processes are studied to improve the efficiency and yield of pharmaceutical manufacturing
Analyse Biochimique
Biochemical Properties
Cladribine plays a significant role in biochemical reactions by interacting with various enzymes, proteins, and other biomolecules. It is phosphorylated intracellularly by deoxycytidine kinase to form 2-chlorodeoxyadenosine triphosphate (Cd-ATP). This active metabolite inhibits ribonucleotide reductase, an enzyme crucial for DNA synthesis, leading to the depletion of deoxyribonucleotide pools . This compound also interacts with DNA polymerase and DNA ligase, further disrupting DNA synthesis and repair processes .
Cellular Effects
This compound exerts profound effects on various cell types and cellular processes. It selectively targets and suppresses lymphocytes, particularly B and T cells, by inducing apoptosis. This is achieved through the incorporation of Cd-ATP into DNA, leading to DNA strand breaks and activation of apoptotic pathways . This compound also affects cell signaling pathways, gene expression, and cellular metabolism by inhibiting ribonucleotide reductase and disrupting the balance of deoxyribonucleotide triphosphates .
Molecular Mechanism
At the molecular level, this compound’s mechanism of action involves its conversion to Cd-ATP, which competes with natural nucleotides for incorporation into DNA. This results in DNA strand breaks and inhibition of DNA synthesis. This compound also inhibits ribonucleotide reductase, reducing the availability of deoxyribonucleotides required for DNA replication . Additionally, this compound induces apoptosis by activating the p53 pathway and releasing cytochrome c from mitochondria .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of this compound change over time. Initially, this compound induces rapid apoptosis in lymphocytes, leading to a significant reduction in lymphocyte count. Over time, the stability and degradation of this compound and its metabolites influence its long-term effects on cellular function. Studies have shown that this compound’s effects can persist for weeks to months, with prolonged lymphocyte depletion and immune modulation .
Dosage Effects in Animal Models
The effects of this compound vary with different dosages in animal models. At low doses, this compound effectively induces apoptosis in lymphocytes without significant toxicity. At higher doses, this compound can cause myelosuppression, immunosuppression, and other adverse effects. Studies have identified threshold doses for this compound’s therapeutic and toxic effects, highlighting the importance of dose optimization in clinical settings .
Metabolic Pathways
This compound is involved in several metabolic pathways. It is phosphorylated by deoxycytidine kinase to form Cd-ATP, which is the active metabolite responsible for its cytotoxic effects. This compound also interacts with mitochondrial deoxyguanosine kinase, contributing to its accumulation in lymphocytes . The metabolism of this compound involves minor cytochrome P450-mediated biotransformation and renal excretion of its metabolites .
Transport and Distribution
This compound is transported and distributed within cells and tissues through nucleoside transporters. Equilibrative nucleoside transporter 1 (ENT1) and concentrative nucleoside transporter 3 (CNT3) facilitate the uptake of this compound into cells . Once inside the cell, this compound is phosphorylated and retained, leading to its accumulation in lymphocytes and other target cells . This compound’s distribution across membranes is also influenced by breast cancer resistance protein (BCRP) and P-glycoprotein .
Subcellular Localization
This compound’s subcellular localization is primarily within the nucleus and mitochondria. The phosphorylated form, Cd-ATP, is incorporated into DNA within the nucleus, leading to DNA strand breaks and apoptosis . This compound also localizes to mitochondria, where it disrupts mitochondrial function and induces the release of cytochrome c, further promoting apoptotic pathways .
Méthodes De Préparation
Voies de Synthèse et Conditions de Réaction : La cladribine peut être synthétisée par le couplage direct du O-protégé 2-désoxy-ribofuranose avec la 2-chloroadénine silylée. Ceci est suivi par la déprotection du nucléoside protégé résultant dans une étape séparée, puis une étape de purification . Le procédé implique la dissolution de la this compound brute dans un solvant protique en présence d’une base pour former une solution. Cette solution est maintenue à une température élevée jusqu’à ce que la quantité d’impuretés de nucléoside protégé ou partiellement protégé soit réduite. La solution est ensuite refroidie pour former et isoler des cristaux de this compound .
Méthodes de Production Industrielle : La production industrielle de la this compound implique des voies de synthèse similaires mais à plus grande échelle. Le procédé garantit une pureté et un rendement élevés, ce qui le rend adapté aux applications pharmaceutiques .
Analyse Des Réactions Chimiques
Types de Réactions : La cladribine subit diverses réactions chimiques, notamment la phosphorylation, la déphosphorylation et l’incorporation dans l’ADN. Elle est relativement résistante à l’oxydation et à la réduction en raison de sa structure stable .
Réactifs et Conditions Courants :
Phosphorylation : Cette réaction est catalysée par l’enzyme désoxycytidine kinase, convertissant la this compound en sa forme triphosphorylée active.
Déphosphorylation : Cette réaction est médiée par la 5’-nucléotidase, qui peut désactiver la this compound dans certains types de cellules.
Principaux Produits : Le principal produit de la phosphorylation de la this compound est le 2-chlorodésoxyadénosine triphosphate, qui est incorporé dans l’ADN et déclenche l’apoptose .
4. Applications de la Recherche Scientifique
La this compound a un large éventail d’applications de recherche scientifique :
Chimie : Elle est utilisée comme composé modèle pour étudier les analogues de nucléosides et leurs interactions avec les enzymes.
Biologie : La this compound est utilisée pour étudier les mécanismes de la déplétion des lymphocytes et de la modulation immunitaire.
Médecine : C’est un médicament essentiel dans le traitement de la leucémie à tricholeucocytes et de la sclérose en plaques. .
Industrie : Les processus de synthèse et de production de la this compound sont étudiés afin d’améliorer l’efficacité et le rendement de la fabrication pharmaceutique
Comparaison Avec Des Composés Similaires
Pentostatin: Another purine analog used to treat hairy cell leukemia.
Fludarabine: A purine analog used in the treatment of chronic lymphocytic leukemia.
Uniqueness of Cladribine: this compound’s resistance to adenosine deaminase and its selective targeting of lymphocytes make it unique among purine analogs. Its ability to be administered orally for multiple sclerosis treatment also sets it apart from other similar compounds .
Propriétés
IUPAC Name |
(2R,3S,5R)-5-(6-amino-2-chloropurin-9-yl)-2-(hydroxymethyl)oxolan-3-ol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C10H12ClN5O3/c11-10-14-8(12)7-9(15-10)16(3-13-7)6-1-4(18)5(2-17)19-6/h3-6,17-18H,1-2H2,(H2,12,14,15)/t4-,5+,6+/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
PTOAARAWEBMLNO-KVQBGUIXSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1C(C(OC1N2C=NC3=C(N=C(N=C32)Cl)N)CO)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C1[C@@H]([C@H](O[C@H]1N2C=NC3=C(N=C(N=C32)Cl)N)CO)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C10H12ClN5O3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID8022828 | |
| Record name | Cladribine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8022828 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
285.69 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Cladribine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014387 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
6.35e+00 g/L | |
| Record name | Cladribine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014387 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Cladribine is structurally related to fludarabine and pentostatin but has a different mechanism of action. Although the exact mechanism of action has not been fully determined, evidence shows that cladribine is phosphorylated by deoxycytidine kinase to the nucleotidecladribine triphosphate (CdATP; 2-chloro-2′-deoxyadenosine 5′-triphosphate), which accumulates and is incorporated into DNA in cells such as lymphocytes that contain high levels of deoxycytidine kinase and low levels of deoxynucleotidase, resulting in DNA strand breakage and inhibition of DNA synthesis and repair. High levels of CdATP also appear to inhibit ribonucleotide reductase, which leads to an imbalance in triphosphorylated deoxynucleotide (dNTP) pools and subsequent DNA strand breaks, inhibition of DNA synthesis and repair, nicotinamide adenine dinucleotide (NAD) and ATP depletion, and cell death. Unlike other antimetabolite drugs, cladribine has cytotoxic effects on resting as well as proliferating lymphocytes. However, it does cause cells to accumulate at the G1/S phase junction, suggesting that cytotoxicity is associated with events critical to cell entry into S phase. It also binds purine nucleoside phosphorylase (PNP), however no relationship between this binding and a mechanism of action has been established., Cladribine is an antimetabolite. The exact mechanism of action in hairy cell leukemia is unknown. Cladribine is resistant to the action of adenosine deaminase (ADA), which deaminates deoxyadenosine to deoxyinosine. The phosphorylated metabolites of cladribine accumulate in cells with a high ratio of deoxycytidine kinase activity to 5' nucleotidase activity (lymphocytes, monocytes ) and are converted to the active triphosphate deoxynucleotide. Intracellular accumulation of toxic deoxynucleotides selectively kills these cells, which become unable to properly repair single-strand DNA breaks, leading to disruption of cell metabolism. In addition, there is some evidence that deoxynucleotides are incorporated into the DNA of dividing cells and impair DNA synthesis. Cladribine also induces apoptosis (a form of programmed cell death in sensitive cells). Cladribine's action is cell cycle-phase nonspecific; cladribine equally affects dividing and resting lymphocytes., Cladribine has immunosuppressant activity ; restoration of lymphocyte subsets after treatment takes at least 6 to 12 months, although clinical immunocompetence is usually restored after about a month. Significant reductions in T and B lymphocytes occur during treatment (both CD4 and CD8 are affected) and CD4 counts recover more slowly after treatment., /Investigators/ have studied the role of caspases and mitochondria in apoptosis induced by 2-chloro-2'-deoxyadenosine (cladribine) in several human leukemic cell lines. Cladribine treatment induced mitochondrial transmembrane potential (DeltaPsi(m)) loss, phosphatidylserine exposure, caspase activation and development of typical apoptotic morphology in JM1 (pre-B), Jurkat (T) and U937 (promonocytic) cells. Western-blot analysis of cell extracts revealed the activation of at least caspases 3, 6, 8 and 9. Co-treatment with Z-VAD-fmk (benzyloxy-carbonyl-Val-Ala-Asp-fluoromethylketone), a general caspase inhibitor, significantly prevented cladribine-induced death in JM1 and Jurkat cells for the first approximately 40 h, but not for longer times. Z-VAD-fmk also partly prevented some morphological and biochemical features of apoptosis in U937 cells, but not cell death. Co-incubation with selective caspase inhibitors Ac-DEVD-CHO (N-acetyl-Asp-Glu-Val-Asp-aldehyde), Ac-LEHD-CHO (N-acetyl-Leu-Glu-His-Asp-aldehyde) or Z-IETD-fmk (benzyloxycarbonyl-Ile-Glu-Thr-Asp-fluoromethylketone), inhibition of protein synthesis with cycloheximide or cell-cycle arrest with aphidicolin did not prevent cell death. Overexpression of Bcl-2, but not CrmA, efficiently prevented death in Jurkat cells. In all cell lines, death was always preceded by Delta Psi(m) loss and accompanied by the translocation of the protein apoptosis-inducing factor (AIF) from mitochondria to the nucleus. These results suggest that caspases are differentially involved in induction and execution of apoptosis depending on the leukemic cell lineage. In any case, Delta Psi(m) loss marked the point of no return in apoptosis and may be caused by two different pathways, one caspase-dependent and the other caspase-independent. Execution of apoptosis was always performed after Delta Psi(m) loss by a caspase-9-triggered caspase cascade and the action of AIF., Cladribine (chlorodeoxyadenosine, 2-CdA), a synthetic purine nucleoside, is an antineoplastic agent. ... The precise mechanism(s) of antileukemic action of cladribine has not been fully elucidated. Cladribine is phosphorylated by deoxycytidine kinase to the nucleotide cladribine triphosphate (CdATP; 2-chloro-2'-deoxyadenosine 5'-triphosphate), which accumulates and is incorporated into DNA in cells such as lymphocytes that have high levels of deoxycytidine kinase and low levels of deoxynucleotidase. High intracellular concentrations of cladribine triphosphate appear to inhibit ribonucleotide reductase, causing an imbalance in triphosphorylated deoxynucleotide (dNTP) pools and subsequent DNA strand breaks, inhibition of DNA synthesis and repair, nicotinamide adenine dinucleotide (NAD) and ATP depletion, and cell death. Incorporation of accumulated cladribine triphosphate into DNA also may contribute to DNA strand breakage and inhibition of DNA synthesis and repair. Unlike other commonly used antineoplastic drugs that affect purine and pyrimidine metabolism, cladribine has cytotoxic effects on resting as well as proliferating lymphocytes and monocytes. | |
| Record name | Cladribine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00242 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | CLADRIBINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7564 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Impurities |
9-(2-deoxy-beta-D-erythro-pentofurnaosyl)-9H-purin-2,6-diamine; 9-(2-deoxy-beta-D-erythro-pentofurnaosyl)-2-methoxy-9H-purin-6-amine; 2-chloro-7H-purin-6-amine; 2-chloro-9-(2-deoxy-alpha-D-erythro-pentofurnaosyl)-9H-purin-6-amine; 2-deoxy-D-erythro-pentofurnaose; 4-methylbenzamide; methyl 4-methylbenzoate | |
| Record name | CLADRIBINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7564 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals from water, softens at 210-215 °C, solidifies and turns brown ... Also reported as crystals from ethanol | |
CAS No. |
4291-63-8 | |
| Record name | Cladribine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=4291-63-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Cladribine [USAN:USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0004291638 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Cladribine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00242 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Cladribine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8022828 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | (2R,3S,5R)-5-(6-Amino-2-chloropurin-9-yl)-2-(hydroxymethyl)oxalan-3-ol | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CLADRIBINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/47M74X9YT5 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | CLADRIBINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7564 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Cladribine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014387 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
220 °C (softens), resolidifies, turns brown and does not melt below 300 °C, 215 °C | |
| Record name | Cladribine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00242 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | CLADRIBINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7564 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Cladribine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014387 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: How does cladribine exert its cytotoxic effects?
A1: this compound is a prodrug that requires intracellular phosphorylation to exert its cytotoxic effects. It enters cells and is phosphorylated primarily by the enzyme deoxycytidine kinase (dCK) to this compound monophosphate. [, ] Subsequent phosphorylation steps by nucleoside monophosphate kinase and nucleoside diphosphate kinase lead to the formation of this compound triphosphate, the active metabolite. [] this compound triphosphate incorporates into DNA, leading to DNA strand breaks and ultimately apoptosis, or programmed cell death. [, , ]
Q2: Why is this compound selectively toxic to lymphocytes?
A2: this compound's selective toxicity is attributed to the high ratio of dCK to 5′-nucleotidase (5′-NT) in lymphocytes compared to other cell types. [, ] While dCK phosphorylates this compound to its active form, 5′-NT dephosphorylates it, reducing its efficacy. [] This balance favors this compound accumulation in lymphocytes, leading to preferential cell death. [, ]
Q3: Does this compound affect both dividing and non-dividing cells?
A3: Yes, this compound is cytotoxic to both dividing and non-dividing lymphoid malignancies, setting it apart from many other drugs. []
Q4: What is the molecular formula and weight of this compound?
A4: The molecular formula of this compound is C10H12ClN5O3. Its molecular weight is 285.69 g/mol.
Q5: Is there spectroscopic data available for this compound?
A5: While specific spectroscopic data isn't provided in the provided research papers, standard characterization techniques like NMR, IR, and mass spectrometry are routinely employed for compounds like this compound.
Q6: What is known about this compound's stability under various conditions?
A6: The provided research primarily focuses on this compound's biological activity and doesn't delve deep into its material compatibility or stability under diverse storage conditions.
Q7: How do structural modifications to this compound influence its activity?
A7: Research indicates that modifying the monophosphate group of this compound can significantly enhance its activity against myeloid cell lines. [] These "protide" analogs demonstrate up to 11-fold increased potency compared to this compound itself. [] This highlights the importance of the monophosphate group in cellular uptake and subsequent conversion to the active triphosphate form. []
Q8: How is this compound metabolized and eliminated from the body?
A8: Information on this compound's specific metabolic pathways and elimination routes is not extensively detailed in the provided research papers.
Q9: What is the duration of this compound's effects on lymphocytes?
A10: this compound induces a long-lasting depletion of lymphocytes, particularly B cells, even after a single course of treatment. [, , ] This sustained effect is attributed to its preferential accumulation in lymphocytes and interference with DNA synthesis and repair mechanisms. [, ] Clinical studies have reported continued disease stabilization in relapsing multiple sclerosis patients for several years after this compound treatment, highlighting its prolonged pharmacodynamic effects. [, , ]
Q10: Has this compound shown efficacy in preclinical models of multiple sclerosis?
A11: While the provided research predominantly focuses on clinical studies, this compound's efficacy has been demonstrated in experimental autoimmune encephalomyelitis (EAE), a common animal model of MS. [] In these models, this compound effectively suppressed inflammatory responses and reduced disease severity, supporting its therapeutic potential in MS. []
Q11: What are the clinical outcomes of this compound treatment in hairy cell leukemia?
A12: this compound has demonstrated remarkable efficacy in treating hairy cell leukemia, achieving complete remissions in the majority of patients after a single course of treatment. [, ] Long-term follow-up studies confirm durable remissions lasting several years, solidifying its position as a first-line treatment option for this disease. [, ]
Q12: Are there biomarkers for predicting response to this compound in multiple sclerosis?
A13: Research has explored the potential of molecular biomarkers for predicting this compound response. A study identified specific genes (PPIF, NHLRC2) and microRNAs (miR-21-5p, miR-30b-5p, miR-30e-5p) associated with this compound treatment in multiple sclerosis patients. [] These findings, while preliminary, suggest that analyzing the expression of these molecules could hold promise as potential biomarkers for treatment response. []
Q13: What are the known mechanisms of resistance to this compound?
A13: Resistance to this compound can arise from various mechanisms, including:
- Decreased nucleoside transport: Reduced cellular uptake of this compound limits its intracellular accumulation and subsequent activation. []
- Deoxycytidine kinase (dCK) deficiency or decreased activity: As dCK is crucial for this compound phosphorylation, its deficiency or reduced activity significantly hinders the formation of the active metabolite. [, ]
- Altered intracellular nucleotide pools: Imbalances in competing nucleotides can affect this compound's incorporation into DNA, reducing its efficacy. []
- Increased drug inactivation by 5′-NT: Elevated levels of 5′-NT can rapidly dephosphorylate this compound monophosphate, hindering the formation of the active triphosphate. []
- Defective apoptosis induction: Even if this compound successfully incorporates into DNA, resistance can occur if downstream apoptotic pathways are compromised. []
Q14: Does cross-resistance exist between this compound and other purine analogs?
A15: Cross-resistance between this compound and other purine analogs, like fludarabine and pentostatin, can occur, particularly in the context of hairy cell leukemia. [, ] This is often attributed to shared mechanisms of resistance, such as reduced dCK activity or increased 5′-NT levels, which affect the activation and efficacy of these drugs. [, ]
Q15: What are the common adverse effects associated with this compound treatment?
A16: this compound, like many chemotherapeutic agents, can cause side effects, with myelosuppression being the most prominent. [, , ] This manifests as a decrease in white blood cells, red blood cells, and platelets, increasing the risk of infections, anemia, and bleeding. [, ] Lymphopenia, a reduction in lymphocytes, is a predictable effect of this compound due to its mechanism of action. [, ] While generally transient, it can increase susceptibility to infections, particularly opportunistic infections. [, ]
Q16: What analytical methods are used to quantify this compound levels?
A16: While the provided research does not specify the exact analytical methods used for this compound quantification, techniques like high-performance liquid chromatography (HPLC) coupled with ultraviolet (UV) or mass spectrometry (MS) detection are commonly employed for analyzing nucleoside analogs in biological samples.
Q17: Are there alternative treatments for hairy cell leukemia and multiple sclerosis?
A17: Yes, alternative treatments exist for both conditions:
- Interferon beta: Injections of interferon beta can reduce relapse rates and slow disease progression. []
- Glatiramer acetate: Daily injections of glatiramer acetate can modify the immune response and reduce relapses. []
- Sphingosine 1-phosphate (S1P) receptor modulators (e.g., fingolimod): These oral medications work by trapping lymphocytes in lymph nodes, reducing their migration to the central nervous system. [, ]
- Monoclonal antibodies (e.g., natalizumab, ocrelizumab): These intravenous infusions target specific immune cells or molecules involved in MS pathogenesis. []
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.