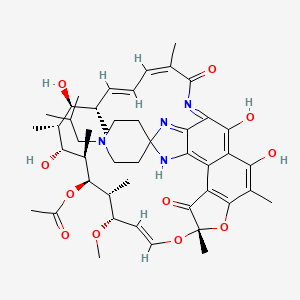
Rifabutine
Vue d'ensemble
Description
Applications De Recherche Scientifique
Treatment of Mycobacterial Infections
Tuberculosis and Non-Tuberculous Mycobacterial Infections
Rifabutin is most notably used in the treatment of tuberculosis (TB), especially for patients with rifampicin-resistant strains. Studies have shown that rifabutin can be effective in treating patients diagnosed with rifampicin-resistant tuberculosis (RR-TB), particularly when used in combination with other antitubercular agents. The Xpert MTB/RIF assay has significantly increased the identification of RR-TB cases, leading to a growing interest in rifabutin-containing regimens as viable therapeutic options for these patients .
Table 1: Treatment Success Rates for Rifabutin vs. Rifampicin
| Study Type | Rifabutin Treatment Success Rate (%) | Rifampicin Treatment Success Rate (%) |
|---|---|---|
| Randomized Trials | 54.7 | 67.5 |
| Observational Studies | Varies | Varies |
This table summarizes the findings from a meta-analysis comparing treatment success rates between rifabutin and rifampicin, indicating that while rifabutin is effective, rifampicin may have a higher overall success rate .
Repurposing for Other Bacterial Infections
Recent studies have explored the potential of repurposing rifabutin for treating infections caused by Staphylococcus aureus, particularly in cases of hospital-acquired infections (HAIs). The structural similarities between rifabutin and rifampicin allow for comparable antibacterial activity against various bacterial strains, making it a candidate for addressing antibiotic resistance challenges .
Case Study: Efficacy Against Staphylococcus aureus
In a recent investigation, researchers found that rifabutin demonstrated significant antibacterial effects against Staphylococcus aureus, suggesting its potential as an alternative treatment option in scenarios where traditional antibiotics fail due to resistance .
Use in HIV-Infected Patients
Rifabutin is frequently employed in HIV-infected patients to prevent opportunistic infections such as Mycobacterium avium complex (MAC). The drug's ability to reduce the systemic exposure of other antiretroviral drugs has been documented, which is crucial for managing drug interactions in this population .
Table 2: Drug Interaction Impact of Rifabutin
| Drug | Effect on Systemic Exposure |
|---|---|
| Maraviroc | Decreased systemic exposure |
| Clarithromycin | Potential protection against resistance |
This table illustrates the effects of rifabutin on other medications commonly used in HIV treatment, highlighting its role in mitigating resistance development .
Pharmacokinetics and Dosage Considerations
Rifabutin exhibits distinct pharmacokinetic properties that influence its therapeutic applications. It has a longer half-life than rifampicin, allowing for less frequent dosing while maintaining effective drug levels in the body. Typical dosages range from 300 mg to 1200 mg per day, depending on the infection being treated and the patient's overall health status .
Table 3: Recommended Dosages for Various Indications
| Indication | Recommended Dosage (mg/day) |
|---|---|
| Tuberculosis | 300 - 600 |
| Mycobacterium avium complex (MAC) | 300 - 1200 |
| Staphylococcus infections | 600 - 900 |
This table provides an overview of recommended dosages for different clinical indications, emphasizing the flexibility of dosing based on specific patient needs .
Adverse Effects and Safety Profile
While generally well-tolerated, rifabutin can lead to adverse effects such as uveitis and gastrointestinal disturbances. Monitoring is essential when prescribing this medication, especially in populations at risk for drug interactions or those receiving multiple therapies .
Case Report: Uveitis Induced by Rifabutin
A documented case highlighted the occurrence of uveitis in a patient undergoing treatment with rifabutin for MAC infection. This emphasizes the need for vigilance and patient education regarding potential side effects during therapy .
Mécanisme D'action
Target of Action
Ansatipin, also known as Rifabutin, is primarily targeted towards DNA-dependent RNA polymerase in susceptible cells . This enzyme plays a crucial role in the transcription process, which is the first step in gene expression.
Mode of Action
Rifabutin interacts with bacterial RNA polymerase, inhibiting its activity . Specifically, it suppresses RNA synthesis, leading to cell death .
Biochemical Pathways
The primary biochemical pathway affected by Rifabutin is the RNA synthesis pathway . By inhibiting DNA-dependent RNA polymerase, Rifabutin suppresses RNA synthesis, which in turn disrupts protein synthesis and leads to cell death .
Result of Action
The primary result of Rifabutin’s action is the prevention of disseminated Mycobacterium avium complex (MAC) disease in patients with advanced HIV infection . By inhibiting RNA synthesis in susceptible cells, Rifabutin prevents the proliferation of the bacteria, thereby helping to control the infection .
Analyse Biochimique
Biochemical Properties
Ansatipin interacts with bacterial RNA polymerase but does not inhibit the mammalian enzyme . It inhibits DNA-dependent RNA polymerase activity in susceptible cells . This interaction with bacterial RNA polymerase is crucial for its role in biochemical reactions .
Cellular Effects
Ansatipin has significant effects on various types of cells and cellular processes. It is used to treat mycobacterium avium complex disease in patients with HIV, indicating its influence on cell function
Molecular Mechanism
The mechanism of action of Ansatipin involves the inhibition of DNA-dependent RNA polymerase activity in susceptible cells . Specifically, it interacts with bacterial RNA polymerase, leading to a suppression of RNA synthesis and cell death .
Méthodes De Préparation
Voies de synthèse et conditions de réaction
La rifabutine est synthétisée par un procédé semi-synthétique à partir de la rifamycine S. Le procédé implique plusieurs étapes, notamment la modification de la structure de la rifamycine S pour introduire le groupe spiropipéridyle . Les conditions de réaction impliquent généralement l'utilisation de solvants organiques et de catalyseurs pour faciliter les transformations chimiques.
Méthodes de production industrielle
La production industrielle de la this compound implique une synthèse à grande échelle utilisant des conditions de réaction optimisées pour garantir un rendement et une pureté élevés. Le processus est soigneusement contrôlé pour maintenir la qualité et la constance du produit final. La production implique plusieurs étapes de purification, notamment la cristallisation et la chromatographie, pour atteindre les niveaux de pureté souhaités .
Analyse Des Réactions Chimiques
Types de réactions
La rifabutine subit diverses réactions chimiques, notamment :
Oxydation : La this compound peut être oxydée pour former différents dérivés.
Réduction : Les réactions de réduction peuvent modifier les groupes fonctionnels de la molécule de this compound.
Substitution : Les réactions de substitution peuvent introduire de nouveaux groupes fonctionnels dans la structure de la this compound.
Réactifs et conditions courants
Les réactifs courants utilisés dans ces réactions comprennent des agents oxydants comme le permanganate de potassium, des agents réducteurs comme le borohydrure de sodium et divers solvants organiques. Les réactions sont généralement effectuées sous des conditions de température et de pression contrôlées pour garantir les résultats souhaités .
Principaux produits formés
Les principaux produits formés à partir de ces réactions comprennent divers dérivés de la this compound ayant des propriétés antimicrobiennes modifiées. Ces dérivés sont souvent étudiés pour leur utilisation potentielle dans le traitement de différentes infections bactériennes .
Applications de la recherche scientifique
La this compound a un large éventail d'applications de recherche scientifique, notamment :
Chimie : Utilisée comme composé modèle pour étudier la synthèse et la modification des antibiotiques ansamycines.
Biologie : Étudiée pour ses effets sur l'ARN polymérase bactérienne et son rôle dans l'inhibition de la croissance bactérienne.
Médecine : Utilisée dans le traitement de la tuberculose et des infections causées par Mycobacterium avium intracellulare.
Mécanisme d'action
La this compound exerce ses effets en inhibant l'ARN polymérase dépendante de l'ADN chez les bactéries. Cette inhibition empêche la transcription de l'ADN bactérien en ARN, arrêtant ainsi la croissance et la réplication des bactéries . Les cibles moléculaires de la this compound comprennent l'enzyme ARN polymérase et divers composants de la machinerie de transcription bactérienne .
Comparaison Avec Des Composés Similaires
Composés similaires
Rifampicine : Un autre antibiotique ansamycine ayant un mécanisme d'action similaire mais un spectre d'activité différent.
Rifapentine : Un dérivé de la rifamycine ayant une demi-vie plus longue et des propriétés pharmacocinétiques différentes.
Rifamycine S : Le composé parent à partir duquel la rifabutine est synthétisée.
Unicité
La this compound est unique en raison de son activité accrue contre le complexe Mycobacterium avium et de sa capacité à induire le cytochrome P-450 3A à des niveaux plus faibles que la rifampicine . Cela fait de la this compound une option précieuse pour le traitement des infections causées par des mycobactéries résistantes et pour une utilisation en thérapie combinée avec d'autres agents antimicrobiens .
Activité Biologique
Rifabutin is a semi-synthetic derivative of rifampicin, primarily used as an antibiotic for treating mycobacterial infections, including tuberculosis and Mycobacterium avium complex (MAC). This article explores the biological activity of rifabutin, focusing on its mechanisms of action, efficacy against various pathogens, and clinical implications supported by research findings and case studies.
Rifabutin exerts its antibacterial effects primarily through the inhibition of DNA-dependent RNA polymerase in susceptible bacteria. This action leads to the suppression of RNA synthesis, ultimately resulting in bacterial cell death. The drug has shown effectiveness against a range of bacteria, including:
- Mycobacterium tuberculosis
- Mycobacterium avium complex
- Acinetobacter baumannii
The selective uptake mechanism involves the FhuE receptor , which is upregulated in nutrient-limited conditions, enhancing rifabutin's activity in specific media such as RPMI supplemented with fetal calf serum (FCS) .
In Vitro Studies
Rifabutin has demonstrated significant in vitro activity against carbapenem-resistant Acinetobacter baumannii (CRAB) isolates. In a study assessing the minimum inhibitory concentration (MIC), rifabutin exhibited potent activity, particularly when cellular uptake was facilitated by FhuE overexpression .
Clinical Efficacy
A meta-analysis comparing rifabutin-based regimens to rifampin-based regimens indicated that the pooled treatment success rate for rifabutin was 54.7% (95% CI: 41.0-67.0%) compared to 67.5% (95% CI: 55.7-77.4%) for rifampin . This suggests that while rifabutin is effective, it may be less successful than rifampin in certain contexts.
Case Studies
- Treatment of MAC : In a cohort study involving patients with disseminated MAC disease, those treated with rifabutin showed varying success rates based on the number of drugs used in their regimen:
- Staphylococcal Prosthetic Infections : A case series reported on the use of rifabutin in patients with staphylococcal infections associated with prosthetic devices. Some patients experienced adverse effects, but overall results supported further investigation into its use as an alternative to rifampin .
Pharmacokinetics and Drug Interactions
Rifabutin is well absorbed orally, with an average bioavailability of approximately 20% and a high protein binding rate of about 85% . It undergoes hepatic metabolism, producing several active metabolites that contribute to its antimicrobial activity . Notably, rifabutin can interact with other medications; for example, it decreases the systemic exposure of maraviroc in HIV-negative adults .
Propriétés
IUPAC Name |
[(7S,9E,11S,12R,13S,14R,15R,16R,17S,18S,19E,21Z)-2,15,17,32-tetrahydroxy-11-methoxy-3,7,12,14,16,18,22-heptamethyl-1'-(2-methylpropyl)-6,23-dioxospiro[8,33-dioxa-24,27,29-triazapentacyclo[23.6.1.14,7.05,31.026,30]tritriaconta-1(32),2,4,9,19,21,24,26,30-nonaene-28,4'-piperidine]-13-yl] acetate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C46H62N4O11/c1-22(2)21-50-18-16-46(17-19-50)48-34-31-32-39(54)28(8)42-33(31)43(56)45(10,61-42)59-20-15-30(58-11)25(5)41(60-29(9)51)27(7)38(53)26(6)37(52)23(3)13-12-14-24(4)44(57)47-36(40(32)55)35(34)49-46/h12-15,20,22-23,25-27,30,37-38,41,48,52-55H,16-19,21H2,1-11H3/b13-12+,20-15+,24-14-,47-36?/t23-,25+,26+,27+,30-,37-,38+,41+,45-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
ZWBTYMGEBZUQTK-PVLSIAFMSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1C=CC=C(C(=O)N=C2C(=C3C(=C4C2=NC5(N4)CCN(CC5)CC(C)C)C6=C(C(=C3O)C)OC(C6=O)(OC=CC(C(C(C(C(C(C1O)C)O)C)OC(=O)C)C)OC)C)O)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C[C@H]1/C=C/C=C(\C(=O)N=C2C(=C3C(=C4C2=NC5(N4)CCN(CC5)CC(C)C)C6=C(C(=C3O)C)O[C@@](C6=O)(O/C=C/[C@@H]([C@H]([C@H]([C@@H]([C@@H]([C@@H]([C@H]1O)C)O)C)OC(=O)C)C)OC)C)O)/C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C46H62N4O11 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Weight |
847.0 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
Minimally soluble (0.19 mg/mL) | |
| Record name | Rifabutin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00615 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Mechanism of Action |
Rifabutin acts via the inhibition of DNA-dependent RNA polymerase in gram-positive and some gram-negative bacteria, leading to a suppression of RNA synthesis and cell death. | |
| Record name | Rifabutin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00615 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
72559-06-9 | |
| Record name | Rifabutin [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0072559069 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Rifabutin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00615 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
A: [] Rifabutin, similar to Rifampicin, acts by inhibiting the bacterial DNA-dependent RNA polymerase, specifically targeting the beta subunit (rpoB) of the enzyme. This inhibition effectively blocks the initiation of RNA synthesis, ultimately leading to bacterial cell death.
ANone: Rifabutin has the molecular formula C46H56N4O11 and a molecular weight of 849.0 g/mol.
A: While the provided research does not include specific spectroscopic data, techniques like nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry have been used to characterize Rifabutin and its metabolites in various studies. []
ANone: The provided research primarily focuses on the pharmacological and pharmacokinetic aspects of Rifabutin. Information regarding its material compatibility with various substances is limited within these studies.
ANone: Rifabutin is primarily known for its antimicrobial properties and is not reported to exhibit significant catalytic activity. Its primary mode of action involves binding to a specific target protein rather than catalyzing chemical reactions.
ANone: While the provided research does not detail specific computational studies, techniques like quantitative structure-activity relationship (QSAR) modeling could be employed to explore the relationship between Rifabutin's structure and its biological activity.
A: [] Structural modifications, particularly around the rifamycin core structure, are known to influence Rifabutin's activity, potency, and selectivity. For example, modifications influencing the interaction with the rpoB binding site can significantly impact its ability to inhibit bacterial RNA polymerase.
A: [] Rifabutin exhibits stability challenges and can degrade under various conditions, potentially affecting its efficacy. Formulation strategies are crucial to enhance its stability, solubility, and bioavailability.
ANone: The provided research primarily focuses on the clinical and pharmacological aspects of Rifabutin. Detailed information regarding SHE regulations and compliance requirements would necessitate consulting relevant regulatory guidelines and agencies.
A: [, ] Rifabutin exhibits low oral bioavailability due to significant first-pass metabolism. It is extensively metabolized, primarily by CYP3A4 enzymes in the liver and intestines. The major active metabolite, 25-O-Desacetyl-Rifabutin, contributes to its antimicrobial activity. Rifabutin is excreted in both urine and feces.
A: [, ] Rifabutin has demonstrated clinical efficacy in preventing and treating Mycobacterium avium complex (MAC) infections, especially in individuals with HIV. Its efficacy against other mycobacterial species and in specific patient populations, like children, is being actively researched. [, ]
A: [, ] In vitro susceptibility testing, typically using broth microdilution methods, is commonly employed to determine the minimum inhibitory concentration (MIC) of Rifabutin against various mycobacterial strains.
A: [] Mouse models, particularly the beige mouse model, have been utilized to evaluate the in vivo activity of Rifabutin against disseminated MAC infection. These models help assess the efficacy of different doses and treatment regimens.
A: [, ] Yes, several clinical trials have evaluated Rifabutin's safety and efficacy for preventing and treating mycobacterial infections, notably in individuals with HIV.
A: [, ] Resistance to Rifabutin primarily arises from mutations in the rpoB gene, encoding the beta subunit of RNA polymerase, the drug's target. These mutations often alter the drug's binding site, reducing its efficacy.
A: [, ] Cross-resistance, particularly with Rifampicin, is a significant concern. Mutations in the rpoB gene can confer resistance to both drugs, limiting treatment options, especially for multidrug-resistant tuberculosis.
ANone: While the provided research does not detail specific drug delivery approaches for Rifabutin, research into targeted delivery strategies, such as liposomal formulations or nanoparticle-based systems, could be explored to enhance its efficacy and potentially minimize side effects.
A: [] While therapeutic drug monitoring of Rifabutin levels is not routinely performed, measuring plasma concentrations could be beneficial in specific clinical situations, such as suspected drug interactions or treatment failures.
A: [] High-performance liquid chromatography (HPLC), often coupled with UV detection or mass spectrometry, is a common method for quantifying Rifabutin and its metabolites in biological matrices like plasma and urine.
ANone: The provided research focuses primarily on the clinical aspects of Rifabutin. Information regarding its environmental fate, persistence, and potential ecotoxicological effects is limited within these studies and would require further investigation.
A: [, ] Rifabutin exhibits limited aqueous solubility, which can affect its dissolution rate and, consequently, its bioavailability. Formulation strategies are crucial to address these challenges and improve its therapeutic efficacy.
A: [] Validation of analytical methods for Rifabutin involves establishing and documenting method performance characteristics such as accuracy, precision, specificity, linearity, range, limit of detection (LOD), and limit of quantification (LOQ). These parameters ensure the reliability and reproducibility of the analytical data.
ANone: The provided research primarily focuses on Rifabutin's antimicrobial activity and pharmacokinetic properties. Information regarding its potential to induce an immune response or elicit hypersensitivity reactions is limited within these studies.
ANone: While the provided research does not extensively explore Rifabutin's interactions with drug transporters, further investigation into its potential to interact with efflux transporters, like P-glycoprotein (P-gp), is warranted. Such interactions could impact its absorption and distribution within the body.
A: [, ] Yes, Rifabutin is known to induce the activity of specific drug-metabolizing enzymes, particularly CYP3A4. This induction can lead to increased metabolism and reduced plasma concentrations of co-administered drugs that are substrates of these enzymes.
ANone: While Rifabutin is used clinically, suggesting a degree of biocompatibility, the provided research primarily focuses on its antimicrobial activity and pharmacokinetic properties. Detailed information regarding its biocompatibility profile and potential for biodegradation is limited within these studies and would require further investigation.
A: [, ] Alternatives to Rifabutin include Rifampicin (although cross-resistance is a concern), macrolides like Clarithromycin and Azithromycin, and fluoroquinolones like Moxifloxacin. The choice of treatment depends on the specific mycobacterial species, drug susceptibility patterns, patient-specific factors, and potential drug interactions.
ANone: The provided research primarily focuses on the clinical aspects of Rifabutin. Information regarding specific guidelines for its disposal and waste management practices is limited within these studies and would necessitate consulting relevant regulatory guidelines and local regulations.
A: [, ] Key milestones include its initial development as a rifamycin derivative, recognition of its activity against MAC, approval for the prevention of disseminated MAC in individuals with HIV, and exploration of its use in combination therapies for various mycobacterial infections.
A: [] Rifabutin research benefits from the contributions of various disciplines, including microbiology, pharmacology, medicinal chemistry, pharmaceutics, and clinical medicine. Understanding its mechanism of action, resistance mechanisms, pharmacokinetic properties, formulation strategies, and clinical efficacy requires a multidisciplinary approach.
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.















