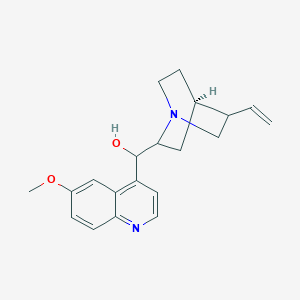
Quinine
Vue d'ensemble
Description
La quinine est un alcaloïde naturel dérivé de l’écorce du quinquina, un arbre originaire des régions andines d’Amérique du Sud. Historiquement, elle a été utilisée comme médicament antipaludéen et est connue pour son goût amer, qui est également utilisé dans l’eau tonique. La this compound a été isolée pour la première fois en 1820 et a joué un rôle crucial dans le traitement du paludisme, en particulier avant l’avènement des médicaments antipaludéens synthétiques .
Mécanisme D'action
La quinine exerce ses effets antipaludéens en interférant avec la capacité du parasite à digérer l’hémoglobine. Elle inhibe la formation d’hémozoïne, un sous-produit toxique de la digestion de l’hémoglobine, ce qui entraîne l’accumulation d’hème libre, qui est toxique pour le parasite. Cela entraîne la mort du parasite du paludisme . De plus, la this compound affecte la membrane musculaire et les canaux sodiques, ce qui explique son utilisation dans le traitement des troubles musculaires .
Composés similaires :
Chloroquine : Un autre médicament antipaludéen qui est plus efficace pour supprimer la croissance des formes sanguines du parasite du paludisme.
Artéméther et luméfantrine : Ces médicaments sont utilisés dans les traitements combinés contre le paludisme et ont des mécanismes d’action différents de ceux de la this compound.
Unicité de la this compound : Le mécanisme d’action unique de la this compound, le développement lent de la résistance et son importance historique en tant que l’un des premiers composés chimiques utilisés pour traiter une maladie infectieuse mettent en évidence son importance. Contrairement aux antipaludéens synthétiques, la this compound est dérivée d’une source naturelle et a une gamme d’applications plus large .
Applications De Recherche Scientifique
Historical Context and Mechanism of Action
Quinine has been utilized for over 400 years as an antimalarial agent. It acts primarily as a blood schizonticide, targeting the Plasmodium species responsible for malaria. The mechanism involves the inhibition of heme polymerase, leading to the accumulation of toxic heme within the parasite's food vacuole, ultimately resulting in its death. Despite its efficacy, this compound's use has declined due to the emergence of more effective and less toxic alternatives, such as artemisinin derivatives.
Antimalarial Applications
This compound remains crucial in treating severe malaria, especially in cases resistant to chloroquine. It is often administered intravenously in life-threatening situations.
Efficacy Data from Clinical Studies
A review of various studies highlights the effectiveness of this compound in different regions with varying resistance patterns:
| Study Site | Year | Sample Size | Treatment Regimen | Cure Rate |
|---|---|---|---|---|
| Thailand | 1984-1985 | 66 children | This compound | 85% |
| Cambodia | 1983 | 119 adults | Mefloquine + SP | 98% |
| Equatorial Guinea | 1999 | 114 children | This compound | 94.5% |
| Cameroon | 2005 | 30 children | This compound | 100% |
These studies illustrate that while this compound is effective, its cure rates can vary significantly based on geographic resistance patterns and treatment regimens .
Treatment of Muscle Cramps
This compound has been explored for its efficacy in treating nocturnal leg cramps. A notable study utilized an n-of-1 trial design involving patients suffering from muscle cramps:
- Participants: 13 patients aged around 75 years
- Method: Randomized crossover design with placebo
- Results: Three patients showed significant improvement with this compound (p < 0.05), while others noted non-significant benefits .
This study underscores this compound's potential role in managing muscle cramps despite its side effects.
Other Therapeutic Uses
Beyond its antimalarial properties, this compound has been investigated for several other applications:
- Antipyretic and Analgesic Effects: Historically used to reduce fever and relieve pain.
- Management of Babesiosis: Effective against the Babesia parasite.
- Potential Role in SARS-CoV-2 Prevention: Although trials were terminated, initial investigations suggested possible antiviral properties .
Adverse Effects and Safety Concerns
This compound is associated with several adverse effects, including:
- Cinchonism: Characterized by tinnitus, headache, and visual disturbances.
- Thrombocytopenia: Documented cases show a significant drop in platelet counts among patients treated with this compound .
- Kidney Injury Risk: Long-term exposure to this compound has been linked to an increased risk of acute kidney injury .
Case Study: this compound-Induced Thrombocytopenia
A randomized controlled trial involving children with severe malaria revealed that approximately 30% of patients treated with this compound experienced a significant drop in platelet count compared to only 10% in those treated with artesunate combination therapy (p = 0.02). Despite this adverse effect, no bleeding incidents were reported .
Case Study: Efficacy in Pregnancy
This compound continues to be a critical treatment option for pregnant women suffering from malaria, particularly during the first trimester when safer alternatives are not available .
Analyse Biochimique
Biochemical Properties
Quinine plays a role in biochemical reactions as an electron carrier . It has the potential to bind to thiol, amine, and hydroxyl groups . These properties make this compound a biologically active compound .
Cellular Effects
This compound is used to treat life-threatening infections caused by chloroquine-resistant Plasmodium falciparum malaria . It acts as a blood schizonticide and also has gametocytocidal activity against P. vivax and P. malariae . As a weak base, it is concentrated in the food vacuoles of P. falciparum .
Molecular Mechanism
It is known that this compound interferes with the parasite’s ability to break down and digest hemoglobin . Consequently, the parasite is poisoned by its own waste product (hemozoin) .
Temporal Effects in Laboratory Settings
It is known that this compound is a stable compound and can be used for quantitative analysis in liquids .
Dosage Effects in Animal Models
It is known that this compound has a narrow therapeutic index, with a large number of side effects at higher doses .
Metabolic Pathways
This compound is metabolized in the liver by the cytochrome P450 system into several metabolites that are excreted in the urine
Transport and Distribution
This compound is rapidly absorbed from the gastrointestinal tract and distributed throughout the body tissues . It is also known to cross the placenta and can be found in the fetus .
Subcellular Localization
Due to its weak base properties, it is known to accumulate in acidic compartments of the cell, such as food vacuoles of the malaria parasite .
Méthodes De Préparation
Voies de synthèse et conditions de réaction : La synthèse totale de la quinine a été une étape importante en chimie organique. La première synthèse totale stéréosélective a été réalisée par Gilbert Stork en 2001. La voie de synthèse implique plusieurs étapes, y compris la formation de quinotoxine, qui est ensuite convertie en this compound par une série de réactions .
Méthodes de production industrielle : La production industrielle de la this compound implique principalement l’extraction de l’écorce du quinquina. L’écorce est récoltée, séchée, puis soumise à une série de processus d’extraction et de purification pour isoler la this compound. La this compound extraite est ensuite convertie en différents sels, tels que le sulfate de this compound ou le chlorhydrate de this compound, pour une utilisation médicinale .
Analyse Des Réactions Chimiques
Types de réactions : La quinine subit diverses réactions chimiques, notamment l’oxydation, la réduction et la substitution. Par exemple, elle peut être oxydée pour former de la quinotoxine, qui peut ensuite subir une réduction pour régénérer la this compound .
Réactifs et conditions courants :
Oxydation : Des réactifs comme l’hypobromite de sodium sont utilisés pour l’oxydation de la this compound en quinotoxine.
Principaux produits : Les principaux produits formés à partir de ces réactions comprennent la quinotoxine et d’autres dérivés, en fonction des conditions de réaction et des réactifs utilisés .
4. Applications de la recherche scientifique
La this compound a un large éventail d’applications dans la recherche scientifique :
Chimie : Elle sert de catalyseur chiral dans la synthèse asymétrique.
Biologie : La this compound est utilisée pour étudier les effets des alcaloïdes sur les systèmes biologiques.
Comparaison Avec Des Composés Similaires
Chloroquine: Another antimalarial drug that is more effective in suppressing the growth of blood forms of the malaria parasite.
Artemether and Lumefantrine: These are used in combination therapies for malaria and have different mechanisms of action compared to quinine.
Uniqueness of this compound: this compound’s unique mechanism of action, slow development of resistance, and its historical significance as one of the first chemical compounds used to treat an infectious disease highlight its importance. Unlike synthetic antimalarials, this compound is derived from a natural source and has a broader range of applications .
Activité Biologique
Quinine, an alkaloid derived from the bark of the cinchona tree, has been historically recognized for its antimalarial properties. Its biological activity extends beyond malaria treatment, encompassing various pharmacological effects, including antimicrobial, analgesic, and potential therapeutic applications in muscle cramps and other conditions. This article delves into the diverse biological activities of this compound, supported by case studies, research findings, and relevant data tables.
This compound acts primarily as a blood schizonticide , targeting the Plasmodium species responsible for malaria. It inhibits heme polymerase, leading to the accumulation of toxic heme within the parasite. This mechanism is crucial in treating chloroquine-resistant strains of Plasmodium falciparum . Additionally, this compound exhibits gametocytocidal activity against P. vivax and P. malariae, making it a versatile agent in malaria management.
Antimicrobial Properties
Recent studies have highlighted this compound's potential as an antimicrobial agent. For instance, a study demonstrated that this compound hydrochloride (Q-HCL) significantly enhances the effectiveness of antimicrobial blue light against Gram-negative bacteria such as Escherichia coli and Pseudomonas aeruginosa. When combined with blue light therapy, Q-HCL at concentrations as low as 0.125 mg/mL resulted in substantial bacterial inactivation (greater than 5-log reductions) .
Table 1: Antimicrobial Efficacy of this compound Hydrochloride
| Bacterial Species | Q-HCL Concentration (mg/mL) | Log Reduction (CFU) | Statistical Significance |
|---|---|---|---|
| E. coli | 0.125 | >5 | P < 0.01 |
| P. aeruginosa | 0.250 | >5 | P < 0.01 |
| A. baumannii | 0.500 | >7 | P < 0.001 |
Case Study: Muscle Cramps
This compound has been evaluated for its efficacy in treating nocturnal leg cramps through an N-of-1 trial involving 13 patients aged around 75 years. The results indicated that three patients experienced significant benefits from this compound (P < 0.05), while six showed non-significant improvements . The trial underscored the variability in patient response to this compound therapy, suggesting that personalized assessments may be necessary for optimal treatment outcomes.
Table 2: Patient Response to this compound Treatment for Leg Cramps
| Patient ID | Cramps Pre-Treatment | Cramps Post-Treatment | Statistical Significance |
|---|---|---|---|
| 1 | Frequent | Rare | Not Significant |
| 2 | Frequent | Occasional | P < 0.05 |
| 3 | Frequent | Rare | P < 0.05 |
Adverse Effects and Toxicity
Despite its therapeutic benefits, this compound is associated with several adverse effects, including drug-induced thrombocytopenia (DIT) and nephrotic syndrome. A case report documented an instance where an elderly female developed nephrotic syndrome after taking this compound for leg cramps; her condition improved rapidly after discontinuation of the drug and initiation of corticosteroid therapy . This highlights the importance of monitoring patients for potential side effects during this compound treatment.
Propriétés
IUPAC Name |
(R)-[(2S,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-(6-methoxyquinolin-4-yl)methanol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19-,20+/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
LOUPRKONTZGTKE-WZBLMQSHSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
COC1=CC2=C(C=CN=C2C=C1)C(C3CC4CCN3CC4C=C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
COC1=CC2=C(C=CN=C2C=C1)[C@H]([C@@H]3C[C@@H]4CCN3C[C@@H]4C=C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C20H24N2O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID0044280 | |
| Record name | Quinine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0044280 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
324.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Quinine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014611 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
In water, 500 mg/L at 15 °C, 1 g dissolves in: 1900 mL water, 760 mL boiling water, 1 g dissolves in: 80 mL benzene (18 mL at 50 °C), 1.2 mL chloroform, 250 mL dry ether, 20 mL glycerol, 0.8 mL alcohol, 1900 mL of 10% ammonia water; almost insoluble in petroleum ether, Soluble in ether, chloroform, carbon disulfide, glycerol, alkalies, and acids (with formation of salts), Sol in pyrimidine, 3.34e-01 g/L | |
| Record name | Quinine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00468 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | QUININE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/2501 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Quinine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014611 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
The theorized mechanism of action for quinine and related anti-malarial drugs is that these drugs are toxic to the malaria parasite. Specifically, the drugs interfere with the parasite's ability to break down and digest hemoglobin. Consequently, the parasite starves and/or builds up toxic levels of partially degraded hemoglobin in itself., Quinine has a local anesthetic action and analgesic, antipyretic, and oxytocic effects. Quinine also has cardiovascular effects similar to those of quinidine. | |
| Record name | Quinine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00468 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | QUININE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/2501 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Triboluminescent, orthorhombic needles from absolute alcohol, Bulky, white, amorphous powder or crystalline alkaloid, CRYSTALS TURN BROWN ON EXPOSURE TO AIR | |
CAS No. |
72402-53-0, 130-95-0, 1407-83-6 | |
| Record name | (8α,9R)-(±)-6′-Methoxycinchonan-9-ol | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=72402-53-0 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Quinine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=130-95-0 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Quinine [BAN:NF] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000130950 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Quinine tannate [USP] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0001407836 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Quinine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00468 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Cinchonan-9-ol, 6'-methoxy-, (8.alpha.,9R)- | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | Quinine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0044280 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Quinine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.004.550 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | QUININE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/A7V27PHC7A | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | QUININE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/2501 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Quinine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014611 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
177 °C (some decomposition), Microcrystalline powder; mp: 57 °C; efflorescent; loses one water molecule in air, two water molecules over sulfuric acid; anhydrous at 125 °C /Quinine trihydrate/, 57 °C | |
| Record name | Quinine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00468 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | QUININE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/2501 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Quinine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014611 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of quinine against malaria?
A1: While the precise mechanism remains incompletely understood, this compound is known to exert its antimalarial effects by interfering with the parasite's ability to metabolize and detoxify heme. [] During its lifecycle within red blood cells, Plasmodium falciparum degrades hemoglobin, releasing toxic heme. This compound accumulates in the parasite's digestive vacuole, forming complexes with heme and preventing its detoxification into hemozoin. This leads to a buildup of toxic heme, ultimately killing the parasite. []
Q2: How does this compound affect muscle cramps?
A2: The mechanism by which this compound relieves muscle cramps is not fully elucidated. Research suggests that it might interfere with the excitability of muscle fibers, reducing their tendency to contract involuntarily and cause cramps. [] Studies have shown that this compound can block acetylcholine-evoked responses in human muscle nicotinic acetylcholine receptors (nAChRs), suggesting a potential mechanism for its anti-cramping effects. []
Q3: Does this compound affect tryptophan levels in cells?
A3: Yes, research indicates that this compound can disrupt tryptophan transport within cells. It inhibits the uptake of tryptophan by associating with the high-affinity tryptophan/tyrosine permease, Tat2p. This association is suppressible by tryptophan, indicating a competitive interaction. The inhibition of tryptophan uptake by this compound can lead to tryptophan starvation in cells. []
Q4: What is the molecular formula and weight of this compound?
A4: The molecular formula of this compound is C20H24N2O2, and its molecular weight is 324.42 g/mol. []
Q5: Are there any spectroscopic techniques used to characterize this compound?
A5: Yes, various spectroscopic techniques, including Fourier transform infrared (FTIR) spectroscopy, nuclear magnetic resonance (NMR) spectroscopy, and mass spectrometry (MS), have been employed to characterize this compound and its derivatives. FTIR helps identify functional groups, while NMR provides insights into the molecule's structure and connectivity. MS confirms the molecular weight and fragmentation patterns, aiding in structural elucidation. [, , ]
Q6: Does this compound possess any catalytic properties?
A6: While not traditionally considered a catalyst, this compound and its derivatives have found applications in asymmetric synthesis as chiral ligands or organocatalysts. Their unique structural features, characterized by multiple chiral centers and a tertiary amine, enable them to influence the stereochemical outcome of reactions. [, ]
Q7: Have there been any computational studies conducted on this compound?
A7: Yes, computational chemistry approaches have been employed to study this compound's interactions with biological targets and to develop quantitative structure-activity relationship (QSAR) models. Molecular docking studies have provided insights into the binding modes of this compound with enzymes and receptors, while QSAR models have helped predict the antimalarial activity of this compound analogs based on their structural features. []
Q8: How do modifications to the this compound structure impact its activity?
A8: SAR studies have revealed that specific structural features are crucial for this compound's activity. The stereochemistry at the C-9 position significantly influences antimalarial potency, with this compound and quinidine exhibiting higher activity than their 9-epi counterparts. Modifications to the quinoline and quinuclidine rings, as well as the hydroxyl group, have also been explored to understand their impact on antimalarial activity, toxicity, and physicochemical properties. [, ]
Q9: How is this compound metabolized in the body?
A9: this compound is primarily metabolized in the liver via oxidative pathways, involving cytochrome P450 enzymes. Major metabolites include 3-hydroxythis compound and 10,11-dihydroxydihydrothis compound. The metabolic profile of this compound can be influenced by factors like genetic polymorphisms in drug-metabolizing enzymes, co-administration of other drugs, and liver function. [, ]
Q10: How does renal function affect this compound elimination?
A10: Renal impairment can significantly impact the elimination of this compound, as it is primarily excreted in the urine. In patients with chronic renal failure, particularly those on hemodialysis, the clearance of free (unbound) this compound is increased, leading to lower plasma concentrations. This altered pharmacokinetic profile necessitates careful dose adjustments to avoid toxicity. []
Q11: What types of in vitro and in vivo models are used to study this compound's activity?
A11: In vitro studies often utilize cultures of Plasmodium falciparum to determine the drug's efficacy in inhibiting parasite growth. Animal models, such as mice infected with malaria, are used to evaluate in vivo efficacy, pharmacokinetic properties, and potential toxicity. Clinical trials in humans are essential for assessing the drug's safety and efficacy in treating malaria. [, ]
Q12: What are the mechanisms of resistance to this compound in malaria parasites?
A12: Resistance to this compound in Plasmodium falciparum is a complex phenomenon, often involving mutations in genes encoding proteins involved in drug transport or drug targets. Mutations in the Pfmdr1 gene, encoding a transmembrane protein involved in drug efflux, have been associated with decreased susceptibility to this compound. []
Q13: What are the potential adverse effects of this compound?
A13: While generally well-tolerated at therapeutic doses, this compound can cause a range of adverse effects, including gastrointestinal disturbances, tinnitus, headache, and visual disturbances. In rare cases, it can lead to serious complications such as hemolytic anemia, thrombocytopenia, and hypoglycemia. Careful monitoring for adverse events is essential, especially in patients with underlying medical conditions. [, , , , ]
Q14: Are there strategies to improve the delivery of this compound to specific targets?
A14: Researchers are exploring various drug delivery approaches to enhance the targeting and efficacy of antimalarial drugs like this compound. Nanoparticle-based delivery systems, for example, hold promise in improving drug solubility, bioavailability, and targeted delivery to infected red blood cells, potentially reducing toxicity and improving therapeutic outcomes. []
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.















