Empagliflozin
Vue d'ensemble
Description
L'empagliflozine est un médicament oral utilisé principalement pour gérer le diabète de type 2. Il appartient à la classe des médicaments appelés inhibiteurs du cotransporteur sodium-glucose 2 (SGLT2). En inhibant le SGLT2, l'empagliflozine réduit la réabsorption du glucose dans les reins, ce qui entraîne une augmentation de l'excrétion du glucose dans les urines. Cela aide à abaisser le taux de sucre dans le sang chez les patients atteints de diabète de type 2 .
Méthodes De Préparation
Voies de synthèse et conditions de réaction : L'empagliflozine est synthétisée par un procédé en plusieurs étapes impliquant plusieurs intermédiaires clés. Une voie de synthèse courante comprend les étapes suivantes :
Formation de l'intermédiaire I : L'étape initiale implique la réaction du 4-chloro-3-(4-(tétrahydrofuran-3-yloxy)benzyl)phényl avec un donneur de glucosyle approprié pour former l'intermédiaire I.
Formation de l'intermédiaire II : L'intermédiaire I subit d'autres réactions, y compris des étapes de protection et de déprotection, pour produire l'intermédiaire II.
Couplage final : L'intermédiaire II est ensuite couplé à un donneur de glucosyle approprié dans des conditions basiques pour former l'empagliflozine
Méthodes de production industrielle : La production industrielle d'empagliflozine implique généralement une synthèse à grande échelle utilisant des conditions de réaction optimisées pour assurer un rendement élevé et une pureté élevée. Le processus comprend :
Reflux : Le mélange réactionnel est reflué pour faciliter la formation du produit souhaité.
Purification : Le produit brut est purifié en utilisant des techniques telles que la recristallisation et la chromatographie pour obtenir l'empagliflozine avec une pureté élevée
Analyse Des Réactions Chimiques
Types de réactions : L'empagliflozine subit diverses réactions chimiques, notamment :
Hydrolyse : Le composé est susceptible à l'hydrolyse, en particulier dans des conditions acides et basiques.
Réactifs et conditions courants :
Dégradation oxydative : Des réactifs tels que le peroxyde d'hydrogène ou d'autres agents oxydants peuvent induire une dégradation oxydative.
Principaux produits formés :
Produits de dégradation oxydative : Différents produits d'oxydation peuvent être formés, en fonction des conditions spécifiques et des réactifs utilisés.
Produits d'hydrolyse : L'hydrolyse peut conduire à la formation de fragments plus petits et de produits de dégradation.
4. Applications de la recherche scientifique
L'empagliflozine a un large éventail d'applications de recherche scientifique, notamment :
Gestion du diabète : L'empagliflozine est principalement utilisée pour gérer le diabète de type 2 en abaissant le taux de sucre dans le sang
Avantages cardiovasculaires : La recherche a montré que l'empagliflozine peut réduire le risque d'événements cardiovasculaires, tels que les crises cardiaques et les accidents vasculaires cérébraux, chez les patients atteints de diabète de type 2
Protection rénale : L'empagliflozine s'est avérée avoir des effets néphroprotecteurs, réduisant le risque de progression de la maladie rénale.
Insuffisance cardiaque : Le composé est également utilisé pour traiter l'insuffisance cardiaque, offrant des avantages au-delà du contrôle du glucose.
5. Mécanisme d'action
L'empagliflozine exerce ses effets en inhibant le cotransporteur sodium-glucose 2 (SGLT2) dans les reins. Cette inhibition réduit la réabsorption du glucose du filtrat glomérulaire, ce qui entraîne une augmentation de l'excrétion du glucose dans les urines. La réduction du taux de glucose dans le sang aide à gérer le diabète de type 2. De plus, l'empagliflozine s'est avérée avoir des effets cardioprotecteurs et néphroprotecteurs, qui seraient liés à des mécanismes tels que la réduction du stress oxydatif et l'amélioration de la fonction endothéliale .
Applications De Recherche Scientifique
Empagliflozin is a relatively new drug that inhibits the sodium–glucose cotransporter 2 (SGLT2), increasing urinary glucose excretion . Originally used to induce a hypoglycemic effect in patients with type 2 diabetes mellitus (T2DM), this compound has demonstrated other beneficial effects, including nephroprotection and as a breakthrough in treating heart failure (HF) . Studies have shown benefits in patients with and without T2DM .
Scientific Research Applications
A study evaluated this compound initiated in-hospital for acute heart failure . The primary outcome was clinical benefit, assessed using a hierarchical composite of death from any cause, the number of heart failure events, time to first heart failure event, or a 5 point or greater difference in change from baseline in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score at 90 days . More patients treated with this compound had clinical benefit compared with placebo . Clinical benefit was seen for both acute de novo and decompensated chronic heart failure and regardless of ejection fraction or diabetes status . this compound was well tolerated, with fewer serious adverse events than placebo . These results extend and complement those of EMPEROR-Reduced and EMPEROR-Preserved by focusing on patients hospitalized for acute heart failure across the range of ejection fraction .
Renal Protection: this compound significantly reduces the relative risk of developing or worsening nephropathy by 39% compared to placebo . The relative risk reduction for progression to macroalbuminuria between the this compound and placebo groups was 38% . The risk of doubling plasma creatinine levels was reduced by 44% in the this compound group, and the relative risk of starting renal replacement therapy (RRT) was significantly 55% lower .
Alzheimer's Disease: A five-drug combination consisting of Tofacitinib, Niraparib, Baricitinib, this compound, and Doxercalciferol can serve as a promising drug combination for AD treatment . this compound, an SGLT2 inhibitor, regulates the insulin signaling pathway linking diabetes and AD . this compound can target EGFR in the MAPK, FOXO, and Rap1 signaling pathways via SLC5A1 .
Improved Cardiac Function: this compound administration to nondiabetic HFrEF patients significantly improves LV volumes, LV mass, LV systolic function, functional capacity, and quality . this compound significantly ameliorated adverse LV remodeling, decreased LV volumes and LV hypertrophy, reduced neurohormonal activation, and improved cardiac systolic function compared with the control group . Moreover, this compound also improved diastolic function in this HFrEF model .
Improved Clinical Stability: this compound reduces the risk of cardiovascular death or hospitalization for heart failure in patients with heart failure and a reduced ejection fraction . this compound reduced the total number of heart failure hospitalizations that required intensive care and that required a vasopressor or positive inotropic drug or mechanical or surgical intervention . Patients assigned to this compound were 20% to 40% more likely to experience an improvement in New York Heart Association functional class and were 20% to 40% less likely to experience worsening of New York Heart Association functional class, with statistically significant effects that were apparent 28 days after randomization and maintained during long-term follow-up .
Data Table
Mécanisme D'action
Empagliflozin exerts its effects by inhibiting the sodium-glucose co-transporter-2 (SGLT2) in the kidneys. This inhibition reduces the reabsorption of glucose from the glomerular filtrate, leading to increased glucose excretion through urine. The reduction in blood glucose levels helps manage type 2 diabetes. Additionally, this compound has been shown to have cardioprotective and nephroprotective effects, which are thought to be mediated through mechanisms such as reducing oxidative stress and improving endothelial function .
Comparaison Avec Des Composés Similaires
L'empagliflozine fait partie de la classe des médicaments inhibiteurs du SGLT2. D'autres composés similaires dans cette classe comprennent :
Canagliflozine : Un autre inhibiteur du SGLT2 utilisé pour gérer le diabète de type 2.
Dapagliflozine : Ce composé est également utilisé pour traiter le diabète de type 2 et a été approuvé pour une utilisation dans l'insuffisance cardiaque.
Ertugliflozine : Un autre inhibiteur du SGLT2 ayant des mécanismes d'action similaires mais des propriétés pharmacocinétiques différentes.
Unicité de l'empagliflozine : L'empagliflozine est unique par sa haute sélectivité pour le SGLT2 par rapport au SGLT1, ce qui contribue à son efficacité et à son profil de sécurité. Il a également été démontré qu'il avait des avantages cardiovasculaires et rénaux importants, ce qui en fait une option précieuse pour les patients atteints de diabète de type 2 et de comorbidités .
Activité Biologique
Empagliflozin is a sodium-glucose cotransporter 2 (SGLT2) inhibitor primarily used in the management of type 2 diabetes mellitus (T2DM). Its biological activity extends beyond glycemic control, showing significant cardiovascular benefits and effects on cardiac metabolism. This article reviews the biological activity of this compound, supported by recent studies, clinical trials, and case studies.
This compound works by inhibiting the SGLT2 protein in the kidneys, leading to reduced glucose reabsorption and increased glucose excretion. This mechanism not only lowers blood glucose levels but also has secondary effects on cardiovascular health.
- Cardiac Energy Metabolism : Recent studies indicate that this compound enhances cardiac energy status by increasing ATP levels in both cytosolic and mitochondrial compartments within cardiomyocytes. In a study involving db/db mice, this compound treatment resulted in a significant increase in mitochondrial ATP levels, which was associated with improved cardiac function during ischemic conditions .
- Cardioprotection : this compound has been shown to provide cardioprotective effects, particularly in models of heart failure. It improves recovery capacity in ischemic-reperfusion scenarios and maintains ATP levels during stress conditions .
Clinical Evidence
This compound's efficacy has been validated through various clinical trials:
- EMPULSE Trial : This double-blind trial evaluated this compound in patients hospitalized for acute heart failure. Results indicated that this compound significantly improved clinical outcomes compared to placebo, with a stratified win ratio of 1.36 (p = 0.0054), demonstrating its potential benefits even when initiated during hospitalization .
- Long-term Cardiovascular Outcomes : In patients with chronic heart failure, this compound reduced the risk of cardiovascular death and heart failure hospitalization. The drug was well tolerated, with serious adverse events occurring less frequently than in the placebo group .
Case Studies
Several case studies highlight the biological activity of this compound:
- Case Study on Cardiac Remodeling : A study focused on patients with T2DM and coronary artery disease found that this compound treatment led to a reduction in left ventricular mass over time, suggesting beneficial remodeling effects on the heart .
- Impact on Diabetic Patients : Research involving diabetic patients showed that this compound effectively reduced fasting plasma glucose and glycated hemoglobin levels while also improving markers of cardiovascular health, such as blood pressure and weight management .
Summary of Findings
The following table summarizes key findings from recent studies regarding the biological activity of this compound:
Propriétés
IUPAC Name |
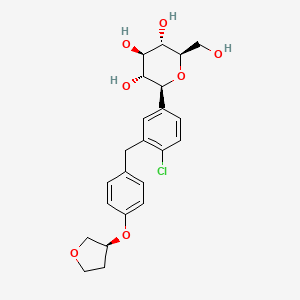
(2S,3R,4R,5S,6R)-2-[4-chloro-3-[[4-[(3S)-oxolan-3-yl]oxyphenyl]methyl]phenyl]-6-(hydroxymethyl)oxane-3,4,5-triol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C23H27ClO7/c24-18-6-3-14(23-22(28)21(27)20(26)19(11-25)31-23)10-15(18)9-13-1-4-16(5-2-13)30-17-7-8-29-12-17/h1-6,10,17,19-23,25-28H,7-9,11-12H2/t17-,19+,20+,21-,22+,23-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
OBWASQILIWPZMG-QZMOQZSNSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1COCC1OC2=CC=C(C=C2)CC3=C(C=CC(=C3)C4C(C(C(C(O4)CO)O)O)O)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C1COC[C@H]1OC2=CC=C(C=C2)CC3=C(C=CC(=C3)[C@H]4[C@@H]([C@H]([C@@H]([C@H](O4)CO)O)O)O)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C23H27ClO7 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID601026093 | |
| Record name | Empagliflozin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID601026093 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
450.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
The vast majority of glucose filtered through the glomerulus is reabsorbed within the proximal tubule, primarily via SGLT2 (sodium-glucose linked co-transporter-2) which is responsible for ~90% of the total glucose reabsorption within the kidneys. Na+/K+-ATPase on the basolateral membrane of proximal tubular cells utilize ATP to actively pump Na+ ions into the interstitium surrounding the tubule, establishing a Na+ gradient within the tubular cell. SGLT2 on the apical membrane of these cells then utilize this gradient to facilitate secondary active co-transport of both Na+ and glucose out of the filtrate, thereby reabsorbing glucose back into the blood – inhibiting this co-transport, then, allows for a marked increase in glucosuria and decrease in blood glucose levels. Empagliflozin is a potent inhibitor of renal SGLT2 transporters located in the proximal tubules of the kidneys and works to lower blood glucose levels via an increase in glucosuria. Empagliflozin also appears to exert cardiovascular benefits - specifically in the prevention of heart failure - independent of its blood glucose-lowering effects, though the exact mechanism of this benefit is not precisely understood. Several theories have been posited, including the potential inhibition of Na+/H+ exchanger (NHE) 1 in the myocardium and NHE3 in the proximal tubule, reduction of pre-load via diuretic/natriuretic effects and reduction of blood pressure, prevention of cardiac fibrosis via suppression of pro-fibrotic markers, and reduction of pro-inflammatory adipokines. | |
| Record name | Empagliflozin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09038 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
864070-44-0 | |
| Record name | Empagliflozin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=864070-44-0 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Empagliflozin [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0864070440 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Empagliflozin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09038 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Empagliflozin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID601026093 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | (2S,3R,4R,5S,6R)-2-(4-chloro-3-(4-((S)-tetrahydrofuran-3-yloxy)benzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | EMPAGLIFLOZIN | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/HDC1R2M35U | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.















