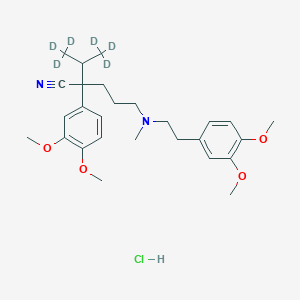
Verapamil-d6 Hydrochloride
- 専門家チームからの見積もりを受け取るには、QUICK INQUIRYをクリックしてください。
- 品質商品を競争力のある価格で提供し、研究に集中できます。
説明
Verapamil-d6 Hydrochloride is a deuterium-labeled version of Verapamil Hydrochloride, a well-known calcium channel blocker. The incorporation of deuterium, a stable isotope of hydrogen, into the molecule does not significantly alter its biochemical properties but allows for differentiation from non-labeled species in analytical techniques such as mass spectrometry . This compound is primarily used in scientific research to study chemical metabolism and kinetics.
準備方法
Synthetic Routes and Reaction Conditions: The synthesis of Verapamil-d6 Hydrochloride involves the incorporation of deuterium into the Verapamil molecule. This process typically includes the use of deuterated reagents and solvents to replace hydrogen atoms with deuterium. The reaction conditions are carefully controlled to ensure the selective incorporation of deuterium without affecting the overall structure of the molecule .
Industrial Production Methods: Industrial production of this compound follows similar principles as its non-deuterated counterpart. The process involves multiple steps, including the synthesis of the Verapamil base, followed by deuteration and subsequent conversion to the hydrochloride salt. The final product is purified using techniques such as crystallization and chromatography to achieve high purity levels .
化学反応の分析
Types of Reactions: Verapamil-d6 Hydrochloride undergoes various chemical reactions, including:
Oxidation: This reaction can be catalyzed by oxidizing agents such as potassium permanganate or hydrogen peroxide.
Reduction: Reduction reactions typically involve reagents like lithium aluminum hydride or sodium borohydride.
Substitution: Nucleophilic substitution reactions can occur with reagents such as sodium methoxide or potassium tert-butoxide.
Common Reagents and Conditions:
Oxidation: Potassium permanganate in acidic conditions.
Reduction: Sodium borohydride in methanol.
Substitution: Sodium methoxide in methanol.
Major Products: The major products formed from these reactions depend on the specific conditions and reagents used. For example, oxidation may yield carboxylic acids, while reduction can produce alcohols .
科学的研究の応用
Pharmacological Applications
Cardiovascular Treatment:
Verapamil-d6 Hydrochloride is primarily used for managing cardiovascular conditions, including:
- Hypertension : It acts as an antihypertensive agent, helping to lower blood pressure by relaxing blood vessels.
- Angina : Effective in treating chronic stable angina and vasospastic angina (Prinzmetal variant).
- Arrhythmias : It is used for the prophylaxis of paroxysmal supraventricular tachycardia and other supraventricular arrhythmias .
Research Insights:
Recent studies have indicated that verapamil can also be beneficial in treating conditions beyond traditional cardiovascular issues. For instance, a recent dose-finding study suggested its potential role as an adjunctive therapy in tuberculosis treatment, enhancing the efficacy of standard antitubercular drugs by inhibiting drug efflux mechanisms in Mycobacterium tuberculosis .
Research Applications
Drug Interaction Studies:
this compound serves as an important tool in pharmacokinetic studies due to its stable isotope labeling. This allows researchers to trace the compound's metabolic pathways and interactions with other drugs. For example, studies have shown that verapamil's pharmacokinetics can be significantly altered when co-administered with rifampin, necessitating careful dose adjustments .
Antimicrobial Research:
The compound has been investigated for its ability to enhance the effectiveness of antibiotics against resistant bacterial strains. By inhibiting efflux pumps in bacteria, this compound can help restore the efficacy of existing antibiotics .
Neuroscience Studies:
In neurological research, verapamil has been explored for its effects on neuronal signaling pathways and potential neuroprotective properties. Its ability to modulate calcium channels makes it a candidate for studying conditions like epilepsy and stroke .
Case Study 1: Tuberculosis Treatment
A clinical trial assessed the safety and efficacy of escalating doses of this compound in patients undergoing rifampin-based tuberculosis therapy. The study demonstrated that higher doses could achieve therapeutic levels without significant adverse effects, suggesting a promising adjunctive role in TB treatment protocols .
Case Study 2: Hypertension Management
In a cohort study involving patients with treatment-resistant hypertension, verapamil-d6 was evaluated alongside other antihypertensives. The results indicated that patients receiving verapamil exhibited improved blood pressure control compared to those on standard therapies alone. This highlights the compound's potential as a valuable addition to hypertension management strategies .
Data Tables
| Application Area | Details |
|---|---|
| Cardiovascular | Treatment of hypertension, angina, arrhythmias |
| Infectious Diseases | Enhances efficacy of antibiotics against resistant strains |
| Neurological | Potential neuroprotective effects; studied in epilepsy and stroke models |
| Drug Interaction | Investigated for interactions with rifampin and other medications |
作用機序
Verapamil-d6 Hydrochloride exerts its effects by inhibiting calcium ions from entering the “slow channels” or select voltage-sensitive areas of vascular smooth muscle and myocardium during depolarization. This action results in the relaxation of coronary vascular smooth muscle and coronary vasodilation, increasing myocardial oxygen delivery in patients with vasospastic angina. Additionally, it slows the automaticity and conduction of the atrioventricular node .
類似化合物との比較
Diltiazem Hydrochloride: Another calcium channel blocker with similar therapeutic uses but different chemical structure.
Nifedipine: A dihydropyridine calcium channel blocker used primarily for hypertension and angina.
Amlodipine: A long-acting dihydropyridine calcium channel blocker used for hypertension and angina.
Uniqueness: Verapamil-d6 Hydrochloride is unique due to its deuterium labeling, which allows for precise analytical studies without significantly altering its pharmacological properties. This makes it an invaluable tool in research settings, particularly for studying the metabolism and kinetics of Verapamil .
生物活性
Verapamil-d6 hydrochloride is a deuterated derivative of verapamil, a well-known calcium channel blocker. This compound is primarily used in pharmacological research due to its unique isotopic labeling, which enhances the tracking and analysis of its biological activity and pharmacokinetics. The following sections provide a comprehensive overview of its biological activity, including mechanisms of action, pharmacokinetics, case studies, and comparative analyses with similar compounds.
- Molecular Formula : C27H32D6N2O4·HCl
- Molecular Weight : Approximately 460.6 g/mol
- Structure : The compound features a core structure similar to verapamil but includes deuterium isotopes that modify its metabolic pathways and analytical properties.
This compound functions primarily as a calcium channel blocker , inhibiting the influx of calcium ions through L-type calcium channels in cardiac and vascular smooth muscle cells. This action leads to several physiological effects:
- Decreased Heart Rate : By reducing calcium influx, it lowers the force of contraction and heart rate.
- Vasodilation : It relaxes vascular smooth muscle, leading to decreased blood pressure.
- Antiarrhythmic Effects : It is effective in treating certain types of cardiac arrhythmias.
Pharmacokinetics
The deuterated form of verapamil allows for enhanced tracking in metabolic studies. Research indicates that deuteration can affect the pharmacokinetic profile by altering the drug's half-life and clearance rates compared to its non-deuterated counterpart. Key pharmacokinetic parameters include:
- Cmax (Maximum Concentration) : The peak plasma concentration observed after administration.
- AUC (Area Under the Curve) : A measure of drug exposure over time.
- t1/2 (Half-Life) : The time taken for the plasma concentration to reduce by half.
Comparative Analysis with Similar Compounds
The following table compares this compound with other calcium channel blockers:
| Compound Name | Structure Similarity | Unique Features |
|---|---|---|
| Verapamil | Core structure identical; lacks deuterium | Widely used as an antiarrhythmic agent |
| Diltiazem | Calcium channel blocker; different aromatic rings | Shorter duration of action compared to verapamil |
| Nifedipine | Calcium antagonist; distinct dihydropyridine structure | Primarily used for hypertension |
| Amlodipine | Similar mechanism; different side chain | Longer half-life and once-daily dosing |
Case Studies and Research Findings
- Pharmacokinetic Study : A recent study administered 40 mg of verapamil hydrochloride to six participants under various conditions (tablet, suspension, crushed) to evaluate absorption differences. Results indicated significant variances in Cmax and AUC depending on the formulation used .
- Drug Interaction Studies : Research has shown that this compound interacts with various drugs, impacting their pharmacokinetics. For instance, it is known to inhibit CYP3A4, a key enzyme involved in drug metabolism, which can lead to increased plasma levels of co-administered drugs .
- Clinical Applications : Verapamil-d6 has been explored for its potential in treating conditions such as hypertension and arrhythmias due to its ability to modulate calcium influx effectively. Its unique isotopic labeling offers insights into metabolic pathways that are crucial for developing safer therapeutic protocols .
特性
IUPAC Name |
2-(3,4-dimethoxyphenyl)-5-[2-(3,4-dimethoxyphenyl)ethyl-methylamino]-2-(1,1,1,3,3,3-hexadeuteriopropan-2-yl)pentanenitrile;hydrochloride |
Source


|
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C27H38N2O4.ClH/c1-20(2)27(19-28,22-10-12-24(31-5)26(18-22)33-7)14-8-15-29(3)16-13-21-9-11-23(30-4)25(17-21)32-6;/h9-12,17-18,20H,8,13-16H2,1-7H3;1H/i1D3,2D3; |
Source


|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
DOQPXTMNIUCOSY-TXHXQZCNSA-N |
Source


|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)C(CCCN(C)CCC1=CC(=C(C=C1)OC)OC)(C#N)C2=CC(=C(C=C2)OC)OC.Cl |
Source


|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
[2H]C([2H])([2H])C(C([2H])([2H])[2H])C(CCCN(C)CCC1=CC(=C(C=C1)OC)OC)(C#N)C2=CC(=C(C=C2)OC)OC.Cl |
Source


|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C27H39ClN2O4 |
Source


|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Weight |
497.1 g/mol |
Source


|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。













