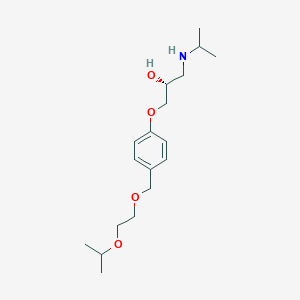
r-(+)-bisoprolol
概要
説明
®-Bisoprolol is a selective beta-1 adrenergic receptor blocker used primarily in the treatment of cardiovascular diseases such as hypertension and heart failure. It is the enantiomer of bisoprolol, meaning it has a specific three-dimensional arrangement that allows it to interact more effectively with biological targets.
準備方法
Synthetic Routes and Reaction Conditions
The synthesis of ®-Bisoprolol typically involves the following steps:
Starting Material: The synthesis begins with the preparation of a chiral intermediate, often derived from natural sources or through asymmetric synthesis.
Key Reactions: The intermediate undergoes several chemical transformations, including alkylation, reduction, and cyclization, to form the desired ®-Bisoprolol.
Reaction Conditions: These reactions are carried out under controlled conditions, such as specific temperatures, pressures, and pH levels, to ensure high yield and purity.
Industrial Production Methods
In industrial settings, the production of ®-Bisoprolol involves large-scale chemical reactors and continuous flow processes. The use of catalysts and optimized reaction conditions helps in achieving efficient production with minimal by-products.
化学反応の分析
Types of Reactions
®-Bisoprolol undergoes various chemical reactions, including:
Oxidation: The compound can be oxidized to form corresponding oxides.
Reduction: Reduction reactions can convert ®-Bisoprolol into its reduced forms.
Substitution: It can undergo substitution reactions where functional groups are replaced by other groups.
Common Reagents and Conditions
Oxidation: Common oxidizing agents include potassium permanganate and hydrogen peroxide.
Reduction: Reducing agents like lithium aluminum hydride are used.
Substitution: Conditions vary depending on the substituent, but typically involve nucleophiles or electrophiles.
Major Products Formed
The major products formed from these reactions depend on the specific reagents and conditions used. For example, oxidation may yield bisoprolol oxides, while reduction may produce bisoprolol alcohols.
科学的研究の応用
Hypertension Management
R-(+)-bisoprolol is widely prescribed for managing hypertension. A systematic review indicated that bisoprolol, especially in combination with hydrochlorothiazide, significantly lowers both systolic and diastolic blood pressure compared to placebo groups. The mean difference in systolic blood pressure was reported as -8.35 mmHg (95% CI: -11.44 to -5.25 mmHg) .
Heart Failure Treatment
The drug is included in guideline-directed medical therapy for heart failure with reduced ejection fraction (HFrEF). It has been shown to reduce mortality and hospitalizations in patients with chronic heart failure . A meta-analysis highlighted that bisoprolol improves cardiac function parameters and reduces adverse outcomes in patients post-myocardial infarction .
Post-Myocardial Infarction Recovery
In patients recovering from myocardial infarction, bisoprolol has demonstrated efficacy in improving functional outcomes and reducing the risk of subsequent cardiac events. Studies have shown significant improvements in left ventricular function when combined with other agents like sacubitril .
Innovative Research and Derivatives
Recent studies have explored novel derivatives of bisoprolol, such as N-acetyl bisoprolol and N-formyl bisoprolol. These derivatives were synthesized through organic reactions and evaluated for their potential as antihypertensive agents using molecular docking techniques. The binding affinities of these derivatives suggest they may offer similar or enhanced therapeutic benefits compared to the parent compound .
Combination Therapy
Research indicates that combining bisoprolol with diuretics like hydrochlorothiazide not only enhances blood pressure control but also improves overall patient outcomes without significantly increasing adverse effects .
Adverse Effects
While generally well-tolerated, bisoprolol can cause side effects such as fatigue, dizziness, and bradycardia. Studies have noted that its selective action minimizes some common side effects associated with non-selective beta-blockers .
Comparative Effectiveness
A comparison between transdermal bisoprolol patches and oral formulations revealed that both methods are effective; however, the patch may offer advantages in terms of patient adherence and side effect profiles .
作用機序
®-Bisoprolol exerts its effects by selectively blocking beta-1 adrenergic receptors, which are primarily found in the heart. This action reduces heart rate and contractility, leading to decreased blood pressure and reduced workload on the heart. The molecular targets include the beta-1 adrenergic receptors, and the pathways involved are related to the sympathetic nervous system.
類似化合物との比較
Similar Compounds
Metoprolol: Another selective beta-1 blocker used for similar indications.
Atenolol: Also a selective beta-1 blocker with similar therapeutic uses.
Propranolol: A non-selective beta-blocker that affects both beta-1 and beta-2 receptors.
Uniqueness
®-Bisoprolol is unique due to its high selectivity for beta-1 adrenergic receptors, which results in fewer side effects related to beta-2 receptor blockade, such as bronchoconstriction. This makes it particularly suitable for patients with respiratory conditions who require beta-blocker therapy.
生物活性
R-(+)-bisoprolol is a highly selective beta-1 adrenergic receptor blocker, primarily used in the treatment of hypertension and heart failure. Its biological activity encompasses a range of pharmacological effects, including cardioprotection, antihypertensive properties, and modulation of metabolic processes. This article synthesizes current research findings on the biological activity of this compound, including data tables and case studies that highlight its efficacy and safety.
Pharmacological Profile
Mechanism of Action
This compound selectively inhibits beta-1 adrenergic receptors, which are predominantly located in the heart and kidneys. This selectivity minimizes the adverse effects commonly associated with non-selective beta-blockers, such as bronchoconstriction. The compound exhibits no intrinsic sympathomimetic activity and has low local anesthetic properties, making it a preferred choice for patients with cardiovascular conditions .
Pharmacokinetics
The pharmacokinetic profile of this compound is characterized by:
- High Bioavailability : Nearly complete enteral absorption with minimal first-pass metabolism.
- Long Half-Life : Extended duration of action allows once-daily dosing.
- Renal Excretion : Approximately 50% of the drug is excreted unchanged via the kidneys .
Efficacy in Clinical Studies
Antihypertensive Effects
This compound has demonstrated significant efficacy in lowering blood pressure in various clinical settings. A meta-analysis comparing bisoprolol with other selective beta-blockers (s-BBs) reported a significant reduction in both systolic and diastolic blood pressure, along with improved heart rate variability .
| Study | Population | Treatment Duration | Outcome |
|---|---|---|---|
| CIBIS-II | CHF Patients | 12 months | Reduced all-cause mortality by 30% |
| Meta-analysis | Hypertensive Patients | 8 weeks | Significant decrease in BP (MD: -3.22 mmHg) |
Cardioprotective Effects
Research indicates that this compound provides cardioprotection during ischemia/reperfusion (I/R) injury. In an animal model, bisoprolol treatment resulted in decreased infarct size and improved cardiac function post-I/R injury by modulating the unfolded protein response (UPR) and reducing inflammation .
Case Studies
CIBIS-II Trial
The CIBIS-II trial was pivotal in establishing the mortality benefits of bisoprolol in chronic heart failure (CHF) patients. The trial was stopped early due to significant findings that showed a reduction in all-cause mortality (hazard ratio: 0.66) compared to placebo .
Impact on Heart Failure Management
In patients with stable CHF, bisoprolol has been shown to improve quality of life and reduce hospitalizations due to heart failure exacerbations. The drug's ability to decrease myocardial oxygen consumption while preserving cardiac output is particularly beneficial for this patient population .
Safety Profile
This compound is generally well-tolerated, with a safety profile that is favorable compared to other beta-blockers. Common side effects include fatigue, dizziness, and bradycardia; however, serious adverse events are rare. Notably, bisoprolol does not significantly alter serum lipid profiles or renal function in most patients .
特性
IUPAC Name |
(2R)-1-(propan-2-ylamino)-3-[4-(2-propan-2-yloxyethoxymethyl)phenoxy]propan-2-ol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C18H31NO4/c1-14(2)19-11-17(20)13-23-18-7-5-16(6-8-18)12-21-9-10-22-15(3)4/h5-8,14-15,17,19-20H,9-13H2,1-4H3/t17-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
VHYCDWMUTMEGQY-QGZVFWFLSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)NCC(COC1=CC=C(C=C1)COCCOC(C)C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC(C)NC[C@H](COC1=CC=C(C=C1)COCCOC(C)C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C18H31NO4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Weight |
325.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
111051-40-2 | |
| Record name | Bisoprolol, (R)- | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0111051402 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | BISOPROLOL, (R)- | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/L68D148Q8N | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Synthesis routes and methods
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。













