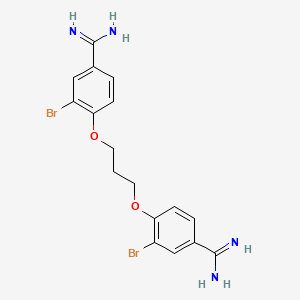
Dibrompropamidine
概要
説明
準備方法
ジブロムプロパミジンの合成には、3-ブロモベンゼン-1-カルボキシミダミドとプロパン-1,3-ジオールの反応が関与します。 反応条件は、通常、エタノールなどの溶媒と触媒の使用を含み、目的の生成物の形成を促進します . 工業生産方法には、同様の反応条件を使用しますが、より高い収率と純度のために最適化された、大規模合成が含まれる場合があります。
化学反応の分析
ジブロムプロパミジンは、次のようなさまざまなタイプの化学反応を起こします。
酸化: この化合物は特定の条件下で酸化され、異なる酸化生成物を形成する可能性があります。
還元: ジブロムプロパミジンは還元されて、対応するアミンまたはその他の還元生成物を形成できます。
置換: ジブロムプロパミジン中の臭素原子は、適切な試薬と条件を使用して、他の官能基で置換できます。
これらの反応に使用される一般的な試薬には、過酸化水素などの酸化剤、水素化ホウ素ナトリウムなどの還元剤、置換反応のための求核剤が含まれます。 形成される主な生成物は、使用される特定の反応条件と試薬によって異なります .
4. 科学研究への応用
ジブロムプロパミジンには、次のいくつかの科学研究への応用があります。
科学的研究の応用
Scientific Research Applications
Dibrompropamidine has been investigated for various applications across different scientific disciplines:
Antimicrobial Research
- Bacterial Infections : Studies have shown that this compound is effective against several strains of bacteria, including those resistant to other antibiotics. Its ability to inhibit microbial growth makes it a candidate for developing new antiseptic agents.
- Fungal Infections : Research indicates potential antifungal properties against specific fungal species, suggesting its utility in treating fungal infections.
- Antiviral Activity : Preliminary studies hint at some antiviral activity, although further research is required to clarify its effectiveness against specific viruses.
Wound Healing
- Recent studies suggest that this compound may promote wound healing and reduce inflammation, making it a subject of interest in dermatological research.
Drug Development
- The compound is being explored for potential applications in drug discovery and repurposing due to its established safety profile and antimicrobial properties .
Case Study 1: Treatment of Microsporidial Keratoconjunctivitis
A clinical study reported successful treatment outcomes for patients with microsporidial keratoconjunctivitis using a combination of topical therapies, including this compound. The study highlighted the compound's role in managing ocular infections effectively, showcasing its therapeutic potential in ophthalmology .
Case Study 2: Antimicrobial Efficacy
In laboratory settings, this compound demonstrated significant antimicrobial activity against various pathogens. A study published in the Journal of Medical Microbiology confirmed its effectiveness against resistant bacterial strains, emphasizing its importance in developing new treatments for infections.
Comparative Data Table
| Application Area | Description | Key Findings |
|---|---|---|
| Antimicrobial Activity | Effective against gram-positive/negative bacteria and certain fungi | Broad-spectrum efficacy |
| Wound Healing | Potential to promote healing and reduce inflammation | Positive results in preliminary studies |
| Drug Development | Investigated for repurposing in pharmaceutical applications | Established safety profile |
| Ophthalmic Use | Used in formulations for treating eye infections | Successful treatment outcomes reported |
作用機序
ジブロムプロパミジンの作用機序は、微生物細胞膜との相互作用を含み、膜の完全性の破壊と微生物の増殖の阻害につながります . この化合物は、微生物における不可欠な細胞プロセスを標的とし、干渉し、最終的にその死につながります。その防腐剤作用に関与する正確な分子経路はまだ調査中です。
6. 類似の化合物との比較
. 類似の化合物には以下が含まれます。
ブロモベンゼン誘導体: これらの化合物は、ベンゼン環上の臭素置換を共有します。
フェノールエーテル: フェノール環にエーテル基が結合した化合物。
カルボキシミダミド: カルボキシミダミド官能基を含む化合物。
これらの類似の化合物と比較して、ジブロムプロパミジンは、その防腐剤特性に寄与する、臭素置換とカルボキシミダミド基の特定の組み合わせのためにユニークです .
類似化合物との比較
. Similar compounds include:
Bromobenzene derivatives: These compounds share the bromine substitution on the benzene ring.
Phenol ethers: Compounds with an ether group attached to a phenol ring.
Carboximidamides: Compounds containing the carboximidamide functional group.
Compared to these similar compounds, dibrompropamidine is unique due to its specific combination of bromine substitution and carboximidamide groups, which contribute to its antiseptic properties .
生物活性
Dibrompropamidine, a compound known for its antimicrobial properties, has garnered attention in various fields of research, particularly in pharmacology and medicinal chemistry. This article delves into the biological activity of this compound, exploring its mechanisms, efficacy against pathogens, and relevant case studies.
Chemical Structure and Properties
This compound is chemically characterized as follows:
- Chemical Formula : CHBrNO
- Molecular Weight : 426.16 g/mol
- CAS Number : 102-36-3
This compound features a dibrominated phenyl group linked to a propamidine backbone, contributing to its biological activity.
This compound exhibits its antimicrobial effects primarily through the inhibition of nucleic acid synthesis and disruption of cellular membranes. The compound targets bacterial cell walls and membranes, leading to cell lysis and death. Its mechanism can be summarized as follows:
- Cell Membrane Disruption : this compound interacts with the phospholipid bilayer of bacterial membranes, increasing permeability.
- Inhibition of Nucleic Acid Synthesis : It interferes with the synthesis of DNA and RNA by targeting key enzymes involved in these processes.
Antimicrobial Efficacy
This compound has demonstrated broad-spectrum antimicrobial activity against various pathogens, including bacteria and fungi. The following table summarizes its effectiveness against selected microorganisms:
| Microorganism | Minimum Inhibitory Concentration (MIC) |
|---|---|
| Staphylococcus aureus | 0.5 - 2 µg/mL |
| Escherichia coli | 1 - 4 µg/mL |
| Candida albicans | 2 - 8 µg/mL |
| Pseudomonas aeruginosa | 4 - 16 µg/mL |
These values indicate that this compound is particularly effective against gram-positive bacteria and certain fungi.
Case Studies and Research Findings
- Topical Applications : A study evaluated the effectiveness of this compound in topical formulations for treating skin infections caused by resistant strains of bacteria. Results showed significant reduction in infection rates when applied twice daily over a two-week period .
- Comparative Studies : In a comparative analysis with other antiseptics such as chlorhexidine, this compound exhibited superior efficacy in preventing biofilm formation on medical devices . This study highlighted its potential use in clinical settings where biofilm-related infections are prevalent.
- Synergistic Effects : Research has indicated that combining this compound with other antimicrobial agents can enhance its effectiveness. A notable study found that when used alongside silver sulfadiazine, the combined treatment reduced bacterial load significantly more than either agent alone .
Safety and Toxicology
While this compound is effective as an antimicrobial agent, safety assessments have revealed potential cytotoxicity at high concentrations. In vitro studies have shown that concentrations above 8 µg/mL can lead to cell death in human keratinocytes . Therefore, careful consideration of dosage is essential for therapeutic applications.
特性
IUPAC Name |
3-bromo-4-[3-(2-bromo-4-carbamimidoylphenoxy)propoxy]benzenecarboximidamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C17H18Br2N4O2/c18-12-8-10(16(20)21)2-4-14(12)24-6-1-7-25-15-5-3-11(17(22)23)9-13(15)19/h2-5,8-9H,1,6-7H2,(H3,20,21)(H3,22,23) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
GMJFVGRUYJHMCO-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1=CC(=C(C=C1C(=N)N)Br)OCCCOC2=C(C=C(C=C2)C(=N)N)Br | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C17H18Br2N4O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID90197918 | |
| Record name | Dibrompropamidine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID90197918 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
470.2 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
496-00-4 | |
| Record name | Dibromopropamidine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=496-00-4 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Dibrompropamidine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000496004 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Dibrompropamidine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB13548 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Dibrompropamidine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID90197918 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | DIBROMPROPAMIDINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/269M3QL74S | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。















