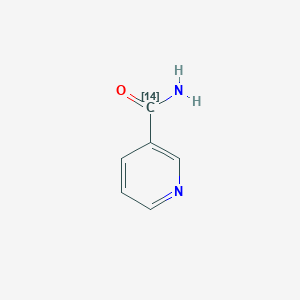
(Carbonyl-14C)nicotinamide
概要
説明
(Carbonyl-14C)nicotinamide is a radiolabeled form of nicotinamide, where the carbonyl carbon is replaced with the radioactive isotope carbon-14. Nicotinamide, also known as niacinamide, is a form of vitamin B3 and is a crucial component of the coenzymes nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP). These coenzymes play essential roles in cellular metabolism and energy production.
準備方法
Synthetic Routes and Reaction Conditions
The synthesis of (Carbonyl-14C)nicotinamide typically involves the incorporation of carbon-14 into the nicotinamide molecule. One common method is the microwave-assisted direct aromatic substitution of 3-bromopyridine with potassium cyanide labeled with carbon-14 (K14CN) as the cyanide source. This reaction is catalyzed by a small amount of tetrabutylammonium bromide and results in the formation of [3-14C]-cyanopyridine. Subsequent microwave-assisted hydrolysis of [3-14C]-cyanopyridine with a mixture of concentrated sulfuric acid and propionic acid yields this compound .
Industrial Production Methods
Industrial production of nicotinamide often involves the use of recombinant Escherichia coli expressing high-molecular-mass nitrile hydratase from Rhodococcus rhodochrous J1. This enzyme catalyzes the conversion of 3-cyanopyridine to nicotinamide. The process involves high cell-density cultivation and substrate fed-batch methods to achieve high yields of nicotinamide .
化学反応の分析
Types of Reactions
(Carbonyl-14C)nicotinamide undergoes various chemical reactions, including:
Oxidation: Nicotinamide can be oxidized to nicotinic acid.
Reduction: Nicotinamide can be reduced to form dihydronicotinamide.
Substitution: Nicotinamide can undergo substitution reactions, such as the replacement of the amide group with other functional groups.
Common Reagents and Conditions
Oxidation: Common oxidizing agents include potassium permanganate and nitric acid.
Reduction: Reducing agents such as sodium borohydride and lithium aluminum hydride are used.
Substitution: Various reagents, including halogens and alkylating agents, can be used for substitution reactions.
Major Products Formed
Oxidation: Nicotinic acid.
Reduction: Dihydronicotinamide.
Substitution: Various substituted nicotinamide derivatives.
科学的研究の応用
Metabolic Studies
1. Pharmacokinetics and Absorption
Studies have demonstrated that (Carbonyl-14C)nicotinamide is effectively absorbed through the skin and gastrointestinal tract. For instance, experiments with topical applications showed that the absorption rate of nicotinamide continues for up to five days, with peak absorption occurring between 48 and 72 hours post-application . Additionally, in vivo studies using animal models have indicated that nicotinamide is rapidly distributed throughout the extracellular fluid after administration .
2. Metabolic Fate in Plants
Research involving the mangrove species Bruguiera gymnorrhiza revealed that this compound supplied to young leaf disks was metabolized into various derivatives, including nicotinic acid. This highlights the compound's role in plant metabolism and its potential applications in agricultural sciences .
Developmental Biology
1. Teratogenic Studies
(Carbony-14C)nicotinamide has been used to investigate its effects on embryonic development. A study demonstrated that administering nicotinamide to pregnant mice significantly reduced urethane-induced malformations such as polydactyly and tail anomalies. The inhibition rates were dose-dependent, indicating a protective effect against teratogenic agents . This suggests potential applications in understanding developmental toxicity and fetal protection mechanisms.
Clinical Applications
1. Dermatological Research
Due to its non-toxic nature at therapeutic concentrations, this compound is explored in dermatological formulations. Clinical trials have shown that topical application can enhance skin barrier function and improve conditions like acne and hyperpigmentation without significant irritation . Its incorporation into cosmetic products is widespread, with concentrations ranging from 0.001% to 3% depending on the formulation type .
2. Cancer Research
The compound has also been studied for its potential anti-carcinogenic properties. In particular, it has been shown to modulate tumor induction by certain carcinogens, suggesting a role in cancer prevention strategies . Studies indicate that nicotinamide may reduce the incidence of tumors induced by substances like streptozotocin, thereby presenting avenues for therapeutic interventions in oncology .
Summary of Key Findings
| Application Area | Key Findings |
|---|---|
| Metabolic Studies | Effective absorption through skin; rapid distribution post-administration |
| Developmental Biology | Reduces urethane-induced malformations; dose-dependent protective effects |
| Clinical Applications | Enhances skin barrier function; non-irritating at therapeutic doses; potential anti-carcinogenic effects |
作用機序
(Carbonyl-14C)nicotinamide exerts its effects primarily through its role as a precursor to NAD and NADP. These coenzymes are involved in various biochemical reactions, including redox reactions, DNA repair, and cell signaling. The molecular targets of nicotinamide include enzymes such as sirtuins, which are involved in regulating cellular processes like aging and inflammation .
類似化合物との比較
Similar Compounds
Nicotinic Acid:
Dihydronicotinamide: A reduced form of nicotinamide.
Nicotinamide Riboside: A precursor to NAD that has gained attention for its potential health benefits.
Uniqueness
(Carbonyl-14C)nicotinamide is unique due to its radiolabeled carbon-14, which allows for precise tracking and quantification in metabolic studies. This makes it particularly valuable in research applications where understanding the metabolic fate of nicotinamide is crucial .
生物活性
Introduction
(Carbonyl-14C)nicotinamide, a radiolabeled form of nicotinamide (vitamin B3), has garnered significant attention in biological research due to its role in metabolic processes and potential therapeutic applications. This article explores the biological activity of this compound, focusing on its metabolic fate, effects on cellular processes, and implications in various biological systems.
Incorporation into Metabolites
Research indicates that this compound is primarily incorporated into pyridine nucleotides, notably NAD (Nicotinamide Adenine Dinucleotide) and NADP (Nicotinamide Adenine Dinucleotide Phosphate). A study involving segments from young and developing leaves of the mangrove plant Bruguiera gymnorrhiza demonstrated that radioactivity from this compound was detected in NAD and trigonelline across all plant parts, with the highest incorporation rates observed in newly emerged stems and young leaves .
Effects of Environmental Stress
The incorporation of this compound into NAD was also assessed under saline conditions. The presence of 500 mM NaCl inhibited both salvage and degradation pathways of nicotinamide metabolism in roots, suggesting that environmental stress can significantly affect its metabolic processing .
Antitumor Activity
Nicotinamide has been shown to possess protective effects against chemically induced malformations and tumorigenesis. In a study on JCL:ICR mice, post-treatment with nicotinamide significantly inhibited urethane-induced malformations, with inhibition levels reaching up to 70% at optimal dosages. Interestingly, when this compound was administered to pregnant mice, it was detected in fetal tissues, indicating direct effects on embryonic development .
Diabetes Intervention
Nicotinamide's role in diabetes prevention has been highlighted through various studies. It has been shown to protect NOD mice from diabetes when administered early in life. The mechanism appears to involve reducing islet inflammation and enhancing NAD levels, which are crucial for cellular metabolism and survival. This protective effect is believed to be mediated through pathways involving sirtuins and protein kinase B (Akt), which are vital for cellular longevity and response to oxidative stress .
Study on Mice
A notable experiment involved treating male C57BL/6J mice on a high-fat diet with nicotinamide riboside (a related compound). Results showed improved glucose tolerance and reduced weight gain, indicating potential benefits for metabolic disorders such as type 2 diabetes. The study also highlighted the neuroprotective effects of nicotinamide against diabetic neuropathy .
Plant Metabolism Study
In another investigation focusing on plant biology, the metabolic incorporation of this compound into various metabolites was analyzed. The findings revealed that this compound plays a critical role in the biosynthesis of important metabolites like trigonelline, which is linked to stress responses in plants .
Data Table: Summary of Biological Activities
特性
IUPAC Name |
pyridine-3-carboxamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C6H6N2O/c7-6(9)5-2-1-3-8-4-5/h1-4H,(H2,7,9)/i6+2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
DFPAKSUCGFBDDF-ZQBYOMGUSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1=CC(=CN=C1)C(=O)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C1=CC(=CN=C1)[14C](=O)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C6H6N2O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID40349349 | |
| Record name | [14C]-Nicotinamide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID40349349 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
124.12 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
10119-18-3 | |
| Record name | 3-Pyridinecaboxamide-14C | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0010119183 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | [14C]-Nicotinamide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID40349349 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Synthesis routes and methods I
Procedure details





Synthesis routes and methods II
Procedure details








Synthesis routes and methods III
Procedure details





Synthesis routes and methods IV
Procedure details




















Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。













