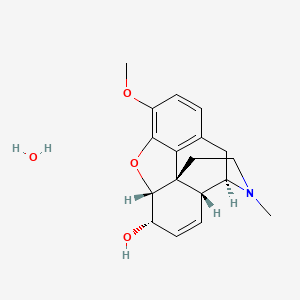
Codeine monohydrate
概要
説明
コデインは、ケシPapaver somniferumの樹液に自然に存在するアルカロイドです。 コデインは、オピオイドであり、モルヒネのプロドラッグであり、主に痛み、咳、および下痢の治療に使用されます . コデインは、鎮痛、鎮咳、および制吐作用のために、医学において広く使用されています .
2. 製法
合成ルートと反応条件: この方法は、モルヒネとトリメチルフェニルアンモニウムクロリドを、アルカリ金属炭酸塩と炭化水素溶媒の存在下で、40°C〜120°Cの温度で反応させることを含みます .
工業生産方法: 工業的には、コデインは通常、アヘンから抽出されるか、モルヒネから合成されます。 抽出プロセスには、アヘンからモルヒネを分離し、メチル化によってコデインに変換することが含まれます .
反応の種類:
酸化: コデインは、酸化されてコデノンを生成することができます。
還元: コデインは、ジヒドロコデインに還元することができます。
一般的な試薬と条件:
酸化: 過マンガン酸カリウムまたは三酸化クロムなどの試薬。
還元: パラジウム触媒の存在下での水素などの試薬。
主要な生成物:
酸化: コデノン。
還元: ジヒドロコデイン。
置換: ノルコデイン.
4. 科学研究における用途
コデインは、科学研究において、幅広い用途を持っています。
化学: 他のオピオイドの合成における基準化合物として使用されます。
生物学: 中枢神経系への影響について研究されています。
医学: 痛みを管理したり、咳を抑制したり、下痢の治療に使用されます.
工業: 様々な医薬品製剤の製造に使用されます.
準備方法
Synthetic Routes and Reaction Conditions: This involves reacting morphine with trimethyl phenyl ammonium chloride in the presence of an alkali metal carbonate and a hydrocarbon solvent at temperatures ranging from 40°C to 120°C .
Industrial Production Methods: In industrial settings, codeine is often extracted from opium or synthesized from morphine. The extraction process involves isolating morphine from opium and then converting it to codeine through methylation .
Types of Reactions:
Oxidation: Codeine can undergo oxidation to form codeinone.
Reduction: Codeine can be reduced to dihydrocodeine.
Substitution: Codeine can undergo substitution reactions, such as N-demethylation to form norcodeine.
Common Reagents and Conditions:
Oxidation: Reagents like potassium permanganate or chromium trioxide.
Reduction: Reagents like hydrogen in the presence of a palladium catalyst.
Substitution: Reagents like sodium hydroxide for N-demethylation.
Major Products:
Oxidation: Codeinone.
Reduction: Dihydrocodeine.
Substitution: Norcodeine.
科学的研究の応用
Pharmacokinetics
Codeine is rapidly absorbed from the gastrointestinal tract, with less than 40% of an orally ingested dose reaching systemic circulation. The peak plasma concentration occurs within 1-2 hours, and its half-life ranges from 2.5 to 3.5 hours . Codeine's solubility and bioavailability are crucial for its effectiveness in various formulations.
Pain Management
Codeine is primarily indicated for the treatment of mild to moderate pain. It is often prescribed in combination with non-opioid analgesics like acetaminophen or non-steroidal anti-inflammatory drugs (NSAIDs) for enhanced efficacy. Despite its common use, recent studies have raised questions about its effectiveness compared to other analgesics .
Cough Suppression
As an antitussive agent, codeine is effective in managing chronic cough associated with various etiologies. Its efficacy can vary based on underlying conditions, and alternatives such as dextromethorphan are sometimes preferred due to superior effectiveness .
Other Indications
- Restless Leg Syndrome : Codeine may be beneficial for refractory cases of restless leg syndrome when other treatments fail.
- Diarrhea : It is occasionally used in palliative care settings to manage persistent diarrhea .
Anticancer Potential
Recent studies have explored the potential anticancer properties of codeine. In vitro studies demonstrated that codeine can reduce cell viability in certain cancer cell lines, suggesting a possible role in cancer prevention or therapy. For instance, codeine showed significant growth inhibition in MCF-7 breast cancer cells and AGS gastric cancer cells when combined with other agents .
Antibacterial Activity
Research has also indicated that codeine complexes exhibit antimicrobial properties against various pathogens, suggesting potential applications beyond traditional uses .
Solubility Studies
Investigations into the solubility of codeine phosphate in supercritical carbon dioxide have been conducted to optimize drug delivery systems. Understanding solubility is essential for enhancing bioavailability and therapeutic effectiveness through micronization techniques .
Pain Management Case Study
A clinical trial involving patients with chronic pain evaluated the efficacy of codeine combined with acetaminophen versus acetaminophen alone. Results indicated that patients receiving the combination experienced greater pain relief without a significant increase in adverse effects.
Cough Management Case Study
A study on patients with chronic cough assessed the effectiveness of codeine syrup compared to dextromethorphan. Findings revealed that while both medications reduced cough frequency, dextromethorphan was preferred by patients due to fewer side effects and better overall satisfaction .
作用機序
コデインは、脳と脊髄のμ-オピオイド受容体に結合することで、効果を発揮します。 この結合は、侵害受容性神経伝達物質の放出を阻害し、鎮痛作用と痛みに対する耐性を高めます . コデインは、肝臓でシトクロムP450 2D6酵素によって代謝され、モルヒネを生成し、鎮痛作用をもたらします .
類似化合物:
モルヒネ: より強力な鎮痛剤であり、依存性のリスクが高いです。
ヒドロコドン: コデインに似ていますが、より強力であり、しばしば重度の痛みに使用されます。
コデインの独自性: コデインは、モルヒネやオキシコドンなどの他のオピオイドと比較して、比較的穏やかな効力と低い依存性リスクを持つ点が特徴です。 安全性の高いプロファイルのため、軽度から中等度の痛みや咳の治療に好まれることが多いです .
類似化合物との比較
Morphine: A more potent analgesic with a higher risk of addiction.
Hydrocodone: Similar to codeine but more potent and often used for severe pain.
Oxycodone: Another potent opioid used for managing moderate to severe pain.
Uniqueness of Codeine: Codeine is unique in its relatively mild potency and lower risk of addiction compared to other opioids like morphine and oxycodone. It is often preferred for treating mild to moderate pain and cough due to its favorable safety profile .
生物活性
Codeine monohydrate, a naturally occurring alkaloid derived from opium poppy, is primarily used for its analgesic and antitussive properties. Its biological activity is largely attributed to its interaction with the central nervous system through opioid receptors, particularly the mu-opioid receptor. This article delves into the pharmacodynamics, pharmacokinetics, metabolism, and clinical implications of this compound, supported by case studies and research findings.
Pharmacodynamics
Mechanism of Action
Codeine exerts its analgesic effects by binding to mu-opioid receptors in the brain and spinal cord, leading to a decrease in the perception of pain. It also interacts with kappa and delta opioid receptors, albeit to a lesser extent. This action results in hyperpolarization of nociceptive neurons, effectively impairing pain signal transmission .
Antitussive Activity
In addition to its analgesic properties, codeine is recognized for its antitussive effects. It suppresses cough reflexes by acting on the cough center in the medulla oblongata . Clinical trials have demonstrated its efficacy in treating cough associated with conditions such as tuberculosis .
Pharmacokinetics
Absorption and Bioavailability
Codeine is rapidly absorbed from the gastrointestinal tract, with peak plasma concentrations typically reached within 1-2 hours post-administration. The bioavailability of codeine ranges from 30% to 40% due to extensive first-pass metabolism in the liver .
Metabolism
Approximately 70-80% of codeine is metabolized in the liver. Key metabolic pathways include:
- O-Demethylation : Converts codeine into morphine (5-10%).
- N-Demethylation : Produces norcodeine (10%).
- Glucuronidation : Forms codeine-6-glucuronide (C6G), which is pharmacologically active .
The enzyme CYP2D6 plays a crucial role in converting codeine to morphine; genetic polymorphisms affecting CYP2D6 can lead to significant variability in individual responses to codeine .
Elimination
The elimination half-life of codeine ranges from 2.5 to 4 hours, with renal excretion being the primary route for its metabolites .
Distribution
Codeine is widely distributed throughout body tissues and can cross the blood-brain barrier as well as the placenta, which raises considerations for use during pregnancy and breastfeeding . The apparent volume of distribution is approximately 3-6 L/kg, indicating extensive tissue binding .
Clinical Implications
Case Studies
- A case study highlighted myopathy and coordination issues linked to prolonged use of codeine linctus, suggesting potential adverse effects associated with chronic administration .
- Another study discussed interactions between codeine and other medications (e.g., carbamazepine), emphasizing the importance of monitoring for adverse reactions when co-administered with other central nervous system depressants .
Summary Table of this compound Characteristics
| Characteristic | Details |
|---|---|
| Chemical Structure | C18H21NO3 (with monohydrate form) |
| Mechanism of Action | Mu-opioid receptor agonist |
| Bioavailability | 30-40% |
| Peak Plasma Concentration | 1-2 hours after oral administration |
| Half-Life | 2.5 - 4 hours |
| Metabolites | Morphine, norcodeine, C6G |
| Primary Metabolism Enzymes | CYP2D6, UGT2B7, UGT2B4 |
| Excretion Route | Renal (urinary) |
特性
CAS番号 |
6059-47-8 |
|---|---|
分子式 |
C18H21NO3 |
分子量 |
299.4 g/mol |
IUPAC名 |
(4R,4aR,7S,7aR,12bS)-9-methoxy-3-methyl-2,4,4a,7,7a,13-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-7-ol |
InChI |
InChI=1S/C18H21NO3/c1-19-8-7-18-11-4-5-13(20)17(18)22-16-14(21-2)6-3-10(15(16)18)9-12(11)19/h3-6,11-13,17,20H,7-9H2,1-2H3/t11-,12+,13-,17-,18-/m0/s1 |
InChIキー |
OROGSEYTTFOCAN-DNJOTXNNSA-N |
SMILES |
CN1CCC23C4C1CC5=C2C(=C(C=C5)OC)OC3C(C=C4)O.O |
異性体SMILES |
CN1CC[C@]23[C@@H]4[C@H]1CC5=C2C(=C(C=C5)OC)O[C@H]3[C@H](C=C4)O |
正規SMILES |
CN1CCC23C4C1CC5=C2C(=C(C=C5)OC)OC3C(C=C4)O |
沸点 |
482 °F at 22 mmHg (sublimes) (NTP, 1992) BP: 250 °C at 22 mm Hg |
Color/Form |
Orthorhombic crystals from water, dilute alcohol, ether Colorless or white crystals or powde |
密度 |
1.32 at 68 °F (NTP, 1992) - Denser than water; will sink |
引火点 |
167 °F (NTP, 1992) |
melting_point |
309 to 313 °F (after drying at 176 °F) (NTP, 1992) 154-156 157.5 °C |
Key on ui other cas no. |
6059-47-8 |
物理的記述 |
Codeine appears as colorless to white crystalline solid or white powder. Sublimes at 284 °F. Odorless. Bitter taste. pH (saturated aqueous solution) 9.8. (NTP, 1992) Solid |
ピクトグラム |
Acute Toxic; Irritant; Health Hazard |
関連するCAS |
41444-62-6 (PO4) |
溶解性 |
less than 1 mg/mL at 70 °F (NTP, 1992) soluble in water Soluble in water Slightly soluble in water Soluble in ethyl ether, benzene, chloroform, toluene; very soluble in ethanol; insoluble in petroleum ether Soluble in alcohol and chloroform 0.577 g/L |
同義語 |
Ardinex Codeine Codeine Phosphate Isocodeine N Methylmorphine N-Methylmorphine |
製品の起源 |
United States |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What structural information about codeine monohydrate, codeine (anhydrous), and dihydrocodeinone is available from the research paper?
A1: The research paper titled "UNIT CELL, SPACE GROUP, AND INDEXED X-RAY DIFFRACTION POWDER DATA FOR CERTAIN NARCOTICS: I. This compound, CODEINE (ANHYDROUS), DIHYDROCODEINONE" [] investigates the crystallographic properties of these compounds. This includes determining the unit cell parameters, space group, and indexed X-ray diffraction powder data. This information is crucial for understanding the arrangement of molecules within the crystal lattice, which can influence properties such as stability, solubility, and bioavailability.
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。















