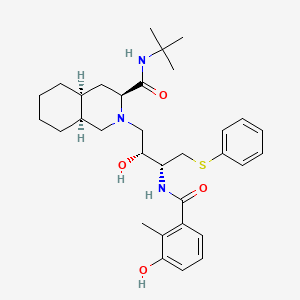
ネルフィナビル
概要
説明
ネルフィナビルは、HIV/AIDSの治療に使用される抗レトロウイルス薬です。 ネルフィナビルは、経口生物学的利用能のあるヒト免疫不全ウイルスHIV-1プロテアーゼ阻害剤であり、1992年に特許を取得し、1997年に医療用として承認されました .
科学的研究の応用
Antiviral Applications
HIV Treatment
Nelfinavir is primarily known as an HIV protease inhibitor, playing a crucial role in highly active antiretroviral therapy (HAART). It works by inhibiting the HIV protease enzyme, which is essential for the maturation of infectious viral particles. This action effectively reduces viral load in patients and improves immune function.
Oncological Applications
Nelfinavir has demonstrated significant antitumor effects across various cancer types through multiple mechanisms:
2.1 Mechanisms of Action
- Inhibition of Signaling Pathways : Nelfinavir suppresses the Akt signaling pathway, which is often dysregulated in cancer cells. By inhibiting this pathway, nelfinavir induces apoptosis and impairs cell proliferation in several cancer types, including small-cell lung cancer (SCLC) and castration-resistant prostate cancer (CRPC) .
- Induction of Unfolded Protein Response (UPR) : In SCLC cells, nelfinavir triggers UPR, leading to cell death. This response is linked to the inhibition of mTOR activation and reduced expression of oncogenic markers like achaete-scute homolog 1 (ASCL1) .
2.2 Case Studies and Clinical Trials
- Small-Cell Lung Cancer : In vitro studies have shown that nelfinavir effectively inhibits SCLC cell proliferation and induces apoptosis at concentrations as low as 10 μM . In vivo experiments with patient-derived xenograft (PDX) models confirmed its efficacy in reducing tumor growth.
- Castration-Resistant Prostate Cancer : Nelfinavir has been identified as a potent inhibitor of S2P cleavage, a process vital for CRPC proliferation. This inhibition leads to increased ER stress and accumulation of inactive precursors of key transcription factors . Clinical trials are being proposed to further explore its therapeutic potential in this context.
Repurposing for Other Diseases
Recent studies suggest that nelfinavir may be effective against other infectious diseases:
- Helminth Infections : A study indicated that nelfinavir shows promise in treating echinococcosis caused by Echinococcus multilocularis. Its repurposing could provide a single-drug therapy option for patients co-infected with HIV and helminths .
Pharmacokinetics and Safety Profile
Nelfinavir's pharmacokinetics have been extensively studied to optimize dosing regimens in HIV-infected populations. Variability in drug metabolism can influence therapeutic outcomes, necessitating careful monitoring during treatment .
Summary Table of Nelfinavir Applications
作用機序
生化学分析
Biochemical Properties
Nelfinavir inhibits the HIV viral proteinase enzyme which prevents cleavage of the gag-pol polyprotein, resulting in noninfectious, immature viral particles . The inhibition of the HIV-1 protease enzyme is a key interaction of Nelfinavir in biochemical reactions .
Cellular Effects
Nelfinavir’s primary cellular effect is the prevention of the formation of infectious HIV-1 particles . By inhibiting the HIV-1 protease enzyme, Nelfinavir prevents the cleavage of the gag-pol polyprotein, which is necessary for the assembly of mature, infectious HIV-1 particles .
Molecular Mechanism
Nelfinavir binds to the protease active site and inhibits the activity of the enzyme . This inhibition prevents cleavage of the viral polyproteins resulting in the formation of immature non-infectious viral particles .
Temporal Effects in Laboratory Settings
In a study analyzing the pharmacokinetic profile of Nelfinavir, it was found that there is a high variability between individuals in Nelfinavir plasma concentrations . The mean average drug plasma concentration was 2.22 ± 1.25 mg/L and the mean AUC during the dosing interval was 17.7 ± 10.0 mg•h/L .
Dosage Effects in Animal Models
While specific dosage effects in animal models are not mentioned in the available literature, it is known that Nelfinavir is used in combination with other antiviral drugs in the treatment of HIV in both adults and children .
Metabolic Pathways
Nelfinavir is metabolized by multiple cytochrome P-450 enzymes including CYP3A and CYP2C19 . One major and several minor oxidative metabolites were found in plasma .
Transport and Distribution
Unchanged Nelfinavir comprised 82-86% of the total plasma radioactivity after a single oral 750 mg dose of 14C-Nelfinavir . This suggests that Nelfinavir is primarily present in its original form in the plasma, indicating its stability during transport and distribution within the body .
Subcellular Localization
The specific subcellular localization of Nelfinavir is not mentioned in the available literature. Given its role as a protease inhibitor, it is likely that Nelfinavir interacts with the HIV-1 protease enzyme in the cytoplasm of the cell, where the assembly of new viral particles takes place .
準備方法
化学反応の分析
ネルフィナビルは、酸化、還元、置換など、様々な化学反応を起こします。 ヒト免疫不全ウイルス1型(HIV-1)に対して活性を有するプロテアーゼ阻害剤です . プロテアーゼ阻害剤は、HIVの酵素であるプロテアーゼを阻害します。この酵素は、感染性HIV-1に見られる個々の機能性タンパク質に、ウイルスポリタンパク質前駆体をプロテオリシス的に切断するために必要とされます . ネルフィナビルは、プロテアーゼ活性部位に結合し、酵素の活性を阻害することで、ウイルスポリタンパク質の切断を阻止し、未熟な非感染性ウイルス粒子の形成を招きます .
科学研究への応用
ネルフィナビルは、幅広い科学研究への応用があります。 成人および小児の両方におけるHIV感染の治療に用いられています . さらに、ネルフィナビルは、COVID-19の治療における潜在的な利用可能性についても調査されています。 軽度から中等度のCOVID-19症例における有効性を評価するための臨床試験が進行中です . ネルフィナビルは、抗がん剤としても有望であり、複数の癌における細胞増殖を阻害し、細胞周期を調節することが示されています . さらに、ネルフィナビルは、レンサ球菌属の連鎖球菌などの様々な細菌における病原性因子の産生を阻害することが明らかになっています .
類似化合物との比較
ネルフィナビルは、その特定の構造と作用機序により、プロテアーゼ阻害剤の中でユニークです。 類似の化合物には、リトナビル、ロピナビル、インジナビルなどの他のプロテアーゼ阻害剤が含まれます . これらの化合物もHIVプロテアーゼ酵素を阻害しますが、薬物動態、副作用、有効性において異なる場合があります . ネルフィナビルは、他のHIVプロテアーゼ阻害剤と比較して、癌に対する有効性が高まる構造的基盤となる可能性のある、ユニークなシス-デカヒドロイソキノリン-2-カルボキサミド部分を持つことが判明しました .
生物活性
Nelfinavir, an HIV protease inhibitor, has garnered attention not only for its antiviral properties but also for its potential anticancer activity. This article explores the biological activity of nelfinavir, detailing its mechanisms of action, clinical applications, and research findings.
Nelfinavir exhibits multiple mechanisms that contribute to its biological activity:
- Proteasome Inhibition : Nelfinavir inhibits proteasome activity, leading to the accumulation of misfolded proteins and inducing endoplasmic reticulum (ER) stress. This process triggers the unfolded protein response (UPR), which is crucial for cellular homeostasis and can lead to apoptosis in cancer cells .
- Disruption of Lipid Membranes : Research indicates that nelfinavir interacts with lipid bilayers in cellular organelles, causing lipid bilayer stress. This disruption affects mitochondrial respiration and transmembrane protein transport, contributing to its anticancer effects .
- Inhibition of Signaling Pathways : Nelfinavir has been shown to suppress the Akt signaling pathway, which is vital for cell survival and metabolism. It also inhibits multiple members of the protein kinase-like superfamily, impacting various biological processes associated with carcinogenesis and metastasis .
Clinical Applications
Nelfinavir's anticancer potential has been evaluated in several clinical settings:
- Multiple Myeloma : In a phase I trial (SAKK 65/08), nelfinavir demonstrated significant activity against multiple myeloma (MM), particularly in patients who were refractory to proteasome inhibitors. The combination of nelfinavir with bortezomib and dexamethasone yielded an overall response rate of 65% in heavily pretreated patients .
- Broad Anticancer Activity : Nelfinavir has shown broad anticancer activity across various preclinical models. Its ability to induce apoptosis and cell cycle arrest positions it as a promising candidate for further investigation in cancer therapy .
Research Findings
Recent studies have elucidated the biological activity of nelfinavir through various experimental approaches:
Table 1: Summary of Key Studies on Nelfinavir
Case Studies
-
Case Study: Nelfinavir in Multiple Myeloma
- A patient with advanced MM received nelfinavir as part of a combination therapy. The treatment resulted in a partial response, highlighting nelfinavir's potential as an effective chemotherapeutic agent in this setting.
-
Case Study: Anticancer Mechanisms
- In vitro studies demonstrated that nelfinavir could induce apoptosis in various cancer cell lines by activating ER stress pathways and disrupting mitochondrial function, suggesting a multifaceted approach to cancer treatment.
特性
IUPAC Name |
(3S,4aS,8aS)-N-tert-butyl-2-[(2R,3R)-2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-phenylsulfanylbutyl]-3,4,4a,5,6,7,8,8a-octahydro-1H-isoquinoline-3-carboxamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C32H45N3O4S/c1-21-25(15-10-16-28(21)36)30(38)33-26(20-40-24-13-6-5-7-14-24)29(37)19-35-18-23-12-9-8-11-22(23)17-27(35)31(39)34-32(2,3)4/h5-7,10,13-16,22-23,26-27,29,36-37H,8-9,11-12,17-20H2,1-4H3,(H,33,38)(H,34,39)/t22-,23+,26-,27-,29+/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
QAGYKUNXZHXKMR-HKWSIXNMSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1=C(C=CC=C1O)C(=O)NC(CSC2=CC=CC=C2)C(CN3CC4CCCCC4CC3C(=O)NC(C)(C)C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC1=C(C=CC=C1O)C(=O)N[C@@H](CSC2=CC=CC=C2)[C@@H](CN3C[C@H]4CCCC[C@H]4C[C@H]3C(=O)NC(C)(C)C)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C32H45N3O4S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
159989-65-8 (monomethane sulfonate (salt)) | |
| Record name | Nelfinavir [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0159989647 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID5035080 | |
| Record name | Nelfinavir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5035080 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
567.8 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Nelfinavir | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014365 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Slightly soluble, 1.91e-03 g/L | |
| Record name | Nelfinavir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00220 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Nelfinavir | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014365 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
HIV viral protease is an important enzyme for HIV maturation and pathogenicity since HIV produces its structural and key proteins in the form of a polyprotein that needs to be cleaved by a protease. HIV protease is synthesized as part of the Gag-pol polyprotein, where Gag encodes for the capsid and matrix protein to form the outer protein shell, and Pol encodes for the reverse transcriptase and integrase protein to synthesize and incorporate its genome into host cells. The Gag-pol polyprotein undergoes proteolytic cleavage by HIV protease to produce 66 molecular species which will assume conformational changes to become fully active. Inhibition of protease, therefore, prevents HIV virion from fully maturing and becoming infective. Nelfinavir is a competitive inhibitor of the HIV protease by reversibly binding to the active site of the enzyme, preventing it from interacting with its substrate to produce mature and infectious viral particles. | |
| Record name | Nelfinavir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00220 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
159989-64-7 | |
| Record name | Nelfinavir | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=159989-64-7 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Nelfinavir [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0159989647 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Nelfinavir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00220 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | nelfinavir | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=747167 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Nelfinavir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID5035080 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | NELFINAVIR | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/HO3OGH5D7I | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Nelfinavir | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014365 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
349.84 °C | |
| Record name | Nelfinavir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00220 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Nelfinavir | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014365 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of Nelfinavir?
A1: Nelfinavir was initially developed as a specific inhibitor of human immunodeficiency virus (HIV) protease. [] It exerts its antiviral activity by binding to the active site of the HIV-1 protease, thereby preventing the cleavage of viral polyproteins and inhibiting viral maturation. []
Q2: How does Nelfinavir's activity extend to anti-cancer effects?
A2: Beyond its antiviral activity, Nelfinavir exhibits pleiotropic effects in various cancer cells. [, ] One key mechanism involves the induction of endoplasmic reticulum (ER) stress, leading to the unfolded protein response (UPR) and ultimately apoptosis (programmed cell death). [, , ] Additionally, Nelfinavir inhibits the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway, a key regulator of cell growth and survival, further contributing to its anti-cancer properties. [, ]
Q3: Nelfinavir has been shown to affect lipid metabolism in cancer cells. Can you elaborate?
A3: Nelfinavir inhibits the activity of Site-2 protease (S2P), an enzyme involved in the regulated intramembrane proteolysis (RIP) of sterol regulatory element binding protein-1 (SREBP-1) and activating transcription factor 6 (ATF6). [] This inhibition disrupts lipid homeostasis, contributing to ER stress and promoting cell death.
Q4: Can you explain the role of Nelfinavir in modulating the unfolded protein response (UPR) and its impact on cancer cells?
A4: Nelfinavir activates the UPR in myeloma cells, leading to the upregulation of UPR-related proteins. [] This activation can overcome proteasome inhibitor resistance often observed in multiple myeloma. [] The exact mechanisms underlying this effect are complex but involve modulation of key UPR sensors and downstream effectors.
Q5: How does Nelfinavir impact the tumor microenvironment?
A5: Nelfinavir downregulates hypoxia-inducible factor 1-alpha (HIF-1α) and vascular endothelial growth factor (VEGF) expression, leading to decreased angiogenesis (formation of new blood vessels). [] This, in turn, increases tumor oxygenation, potentially enhancing the efficacy of radiotherapy. []
Q6: What is the role of oxidative stress in Nelfinavir's anticancer activity?
A6: Nelfinavir has been shown to increase reactive oxygen species (ROS) production in breast cancer cells, leading to disruption of the Akt/HSP90 complex and subsequent Akt degradation, ultimately contributing to cell death. [] This ROS-dependent mechanism appears to be selective for cancer cells, sparing normal breast cells. []
Q7: What is the molecular formula and weight of Nelfinavir?
A7: Nelfinavir mesylate, the salt form commonly used in formulations, has the molecular formula C32H45N3O4S • CH4O3S and a molecular weight of 663.87 g/mol.
Q8: Have any computational studies been performed on Nelfinavir?
A8: Yes, computational methods have been used to develop a pharmacophore model for Nelfinavir interaction with the multidrug resistance protein 4 (MRP4/ABCC4). [] This model revealed that Nelfinavir shares a binding site with chemotherapeutic agents like adefovir and methotrexate, highlighting its potential as both a substrate and inhibitor of this transporter. []
Q9: What is the typical route of administration and absorption profile of Nelfinavir?
A10: Nelfinavir is administered orally. [, , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , ] Food significantly affects its absorption, with a high-calorie meal required to achieve optimal plasma concentrations. []
Q10: How is Nelfinavir metabolized in the body?
A11: Nelfinavir undergoes extensive metabolism in the liver, primarily by cytochrome P450 (CYP) enzymes, mainly CYP3A4 and CYP2C19. [, ] The major metabolite, Nelfinavir hydroxy-t-butylamide (M8), exhibits potent antiviral activity. [, , ]
Q11: What factors can influence the pharmacokinetics of Nelfinavir?
A11: Various factors can influence Nelfinavir pharmacokinetics, including age, co-administered medications, and liver function. For instance:
- Age: Children, especially infants, exhibit different pharmacokinetic profiles compared to adults, generally requiring higher doses to achieve similar drug exposure. [, , ]
- Co-administered drugs: Co-administration with other protease inhibitors can lead to significant drug interactions, altering the plasma concentrations of both drugs. [, , ] Similarly, drugs that induce or inhibit CYP3A4 can impact Nelfinavir metabolism. []
- Liver disease: Liver disease, especially moderate to severe, can impair Nelfinavir metabolism, leading to lower M8 formation and requiring dose adjustments. []
Q12: How do Nelfinavir plasma concentrations relate to its efficacy in treating HIV?
A13: Studies suggest a correlation between Nelfinavir plasma concentrations and virological treatment success in HIV-infected individuals. Patients with low Nelfinavir levels are at an increased risk of virologic failure. [] This finding highlights the importance of therapeutic drug monitoring to optimize treatment outcomes.
Q13: What types of in vitro models have been used to study Nelfinavir?
A14: Various in vitro models, including cell lines derived from different cancer types (e.g., breast, lung, ovarian, multiple myeloma) have been used to investigate Nelfinavir's anti-cancer effects. [, , , , , , ] These studies have provided insights into its mechanisms of action, such as induction of apoptosis, ER stress, and inhibition of specific signaling pathways. [, , , , , ]
Q14: What in vivo models have been employed to evaluate Nelfinavir's anti-cancer potential?
A15: Nelfinavir's in vivo efficacy has been assessed in various animal models, including mouse xenograft models of different cancer types. [, , , ] These studies have demonstrated that Nelfinavir can inhibit tumor growth in vivo, often in conjunction with markers of ER stress, autophagy, and apoptosis. [, , , ]
Q15: Have any clinical trials been conducted with Nelfinavir in cancer patients?
A15: Yes, several clinical trials have explored Nelfinavir's potential as an anti-cancer agent in humans. For instance:
- Phase I trials: These trials have primarily focused on establishing the maximum tolerated dose (MTD) and safety profile of Nelfinavir in patients with advanced solid tumors. [] Results indicate that Nelfinavir is generally well-tolerated at doses higher than those used for HIV treatment. []
- Phase I/II trials: A trial combining Nelfinavir with bortezomib in patients with advanced hematologic malignancies demonstrated promising activity, particularly in bortezomib-refractory multiple myeloma. []
Q16: What are the primary HIV protease mutations associated with Nelfinavir resistance?
A17: The D30N mutation is the most common primary resistance mutation selected for by Nelfinavir. [, ] While it confers minimal cross-resistance to other protease inhibitors, the emergence of L90M, often accompanied by secondary mutations, can lead to broad cross-resistance within the protease inhibitor class. []
Q17: How does Nelfinavir resistance impact subsequent treatment options for HIV?
A18: Interestingly, despite the emergence of D30N, many patients experiencing virologic failure on a Nelfinavir-containing regimen can successfully switch to another protease inhibitor and achieve viral suppression. [, ] This observation suggests that the development of cross-resistance, while possible, is not inevitable.
Q18: Is there any evidence of cross-resistance between Nelfinavir and other anti-cancer agents?
A19: While Nelfinavir demonstrates synergism with some chemotherapeutic agents, like bortezomib, [, ] specific information regarding cross-resistance patterns with other anti-cancer drugs is limited within the provided research papers.
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。















