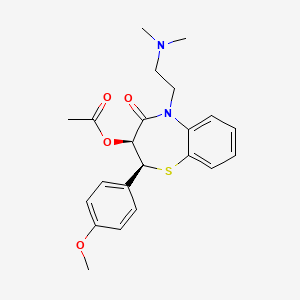
ジルチアゼム
概要
説明
Diltiazem is a benzothiazepine derivative that functions as a calcium channel blocker. It is primarily used to treat cardiovascular conditions such as hypertension, chronic stable angina, and certain heart arrhythmias. Diltiazem works by inhibiting the influx of calcium ions into cardiac and vascular smooth muscle cells, leading to vasodilation and reduced cardiac workload .
作用機序
ジルチアゼムは、心臓および血管平滑筋細胞のL型カルシウムチャネルを遮断することでその効果を発揮します。この阻害は、脱分極中のカルシウムイオンの流入を減らし、平滑筋の弛緩と血管拡張につながります。 細胞内カルシウムレベルの低下は、心筋の収縮力を低下させ、酸素需要を減らすため、狭心症の症状を軽減し、血圧を低下させます .
類似化合物:
ベラパミル: 作用機序は似ていますが、化学構造が異なる別のカルシウムチャネルブロッカーです。
比較:
ジルチアゼム対ベラパミル: 両方の化合物は、心臓および血管平滑筋細胞におけるカルシウムの流入を阻害しますが、ジルチアゼムは心臓組織と血管組織の両方に対して中間的な特異性を持ち、ベラパミルは主に心筋を標的とします.
ジルチアゼム対アムロジピン: ジルチアゼムとアムロジピンはどちらも血管拡張を引き起こしますが、アムロジピンは半減期が長く、主に血管平滑筋に影響を与えるため、長期間の血圧管理に適しています.
ジルチアゼムの独自の、心臓および血管平滑筋の両方を標的とする中間的な特異性により、さまざまな心血管疾患に対する汎用性があり、効果的な治療薬となっています。
科学的研究の応用
Cardiovascular Applications
Hypertension Management
Diltiazem effectively lowers blood pressure in patients with essential hypertension. A significant study, the NORDIL trial, demonstrated that diltiazem reduced both systolic and diastolic blood pressure significantly compared to diuretics and beta-blockers. Specifically, the reduction in diastolic blood pressure was 20.3 mmHg in the diltiazem group versus 18.7 mmHg in the diuretic/beta-blocker group, with a notable 25% reduction in fatal and non-fatal strokes observed in hypertensive patients treated with diltiazem .
Angina Pectoris
Diltiazem is utilized for treating stable, unstable, and variant angina. It works by increasing coronary blood flow and decreasing myocardial oxygen consumption. Clinical studies have shown that diltiazem improves exercise tolerance in patients with chronic stable angina by reducing heart rate and contractility .
Atrioventricular Block
Recent case studies highlight diltiazem's effectiveness in treating complete atrioventricular block caused by coronary artery spasm. Administration of intravenous diltiazem restored sinus rhythm and relieved chest pain in a patient with this condition .
Off-Label Uses
Cluster Headaches and Migraines
Diltiazem has been prescribed off-label for the prophylaxis of cluster headaches and migraines. Research indicates that calcium channel blockers may help manage these conditions by modulating vascular responses .
Cocaine Cravings
Emerging studies suggest that diltiazem may reduce cocaine cravings in animal models, potentially due to its effects on dopaminergic signaling pathways in the brain .
Adverse Effects and Rare Reactions
While diltiazem is generally well-tolerated, it can cause adverse effects such as photosensitivity and hyperpigmentation. A case report documented a rare instance of photosensitivity in an elderly patient, emphasizing the need for awareness regarding this potential reaction .
Research and Drug Repurposing
Pharmacokinetics and Pharmacodynamics
Diltiazem's pharmacokinetic profile indicates that it is metabolized primarily by cytochrome P450 enzymes, particularly CYP3A4, which can influence its interactions with other medications . Understanding these interactions is crucial for optimizing therapeutic regimens involving diltiazem.
Data Summary Table
生化学分析
Biochemical Properties
Diltiazem primarily works by inhibiting the calcium influx into cardiac and vascular smooth muscle during depolarization . It interacts with the alpha-1 subunit of L-type calcium channels, displaying an intermediate specificity to target both the cardiac and vascular smooth muscle .
Cellular Effects
Diltiazem has a profound effect on various types of cells and cellular processes. It relaxes the blood vessels, lowers blood pressure, and increases the supply of blood and oxygen to the heart while reducing its workload . In breast cancer cells, diltiazem has been shown to decrease colony formation and cell migration .
Molecular Mechanism
Diltiazem exerts its effects at the molecular level primarily by inhibiting the calcium influx into cardiac and vascular smooth muscle during depolarization . It binds to the alpha-1 subunit of L-type calcium channels in a fashion somewhat similar to verapamil, another nondihydropyridine (non-DHP) calcium channel blocker .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of Diltiazem can change over time. For instance, it has been shown to produce increases in exercise tolerance, probably due to its ability to reduce myocardial oxygen demand . In cases of overdose, it can lead to severe conditions like hypotension, acidosis, anuria, type 1 respiratory failure, and persistent hypotension .
Dosage Effects in Animal Models
In hypertensive animal models, Diltiazem reduces blood pressure and increases urinary output and natriuresis without a change in the urinary sodium/potassium ratio . The effects of Diltiazem can vary with different dosages in animal models.
Metabolic Pathways
Diltiazem is extensively metabolized in the liver via several pathways including N- and O-demethylation (via cytochrome P450), deacetylation (via plasma and tissue esterases), in addition to conjugation (via sulfation and glucuronidation) .
Transport and Distribution
Diltiazem is well distributed in the body with an apparent volume of distribution of approximately 305 L . It is extensively metabolized in the liver with a systemic clearance of approximately 65 L/h .
Subcellular Localization
Given its mechanism of action, it can be inferred that Diltiazem primarily localizes in the cell membrane where it interacts with L-type calcium channels to exert its effects .
準備方法
合成経路と反応条件: ジルチアゼムは、さまざまな化学中間体の反応を含む多段階プロセスを通じて合成できます。 一般的な合成経路の1つは、2-(ジメチルアミノ)エチルクロリドと2-(4-メトキシフェニル)チオ酢酸を縮合させて中間体を形成し、その後環化させてジルチアゼムを生成する方法です .
工業生産方法: 工業的には、ジルチアゼム塩酸塩は、ジルチアゼムを水に溶解し、塩酸を加えて塩酸塩を形成することで調製されます。 次に、溶液をろ過し、濃縮し、結晶化して純粋なジルチアゼム塩酸塩を得ます .
化学反応の分析
反応の種類: ジルチアゼムは、加水分解、酸化、還元など、さまざまな化学反応を起こします。 注目すべき反応の1つは、ジルチアゼムが酸性または塩基性条件下で加水分解されて脱アセチルジルチアゼムを生成することです .
一般的な試薬と条件:
加水分解: 酸性または塩基性条件
酸化: 過酸化水素などの酸化剤
還元: 水素化ホウ素ナトリウムなどの還元剤
主要な生成物:
脱アセチルジルチアゼム: 加水分解によって生成されます
N-脱メチルジルチアゼム: N-脱メチル化によって生成されます
4. 科学研究への応用
ジルチアゼムは、科学研究において幅広い応用範囲を持っています:
化学: クロマトグラフィー法の開発のための分析化学における標準化合物として使用されます.
生物学: さまざまな細胞タイプにおけるカルシウムイオンチャネルに対するその効果について研究されています。
類似化合物との比較
Verapamil: Another calcium channel blocker with a similar mechanism of action but a different chemical structure.
Amlodipine: A dihydropyridine calcium channel blocker that primarily acts on vascular smooth muscle.
Comparison:
Diltiazem vs. Verapamil: Both compounds inhibit calcium influx in cardiac and vascular smooth muscle cells, but diltiazem has an intermediate specificity for both cardiac and vascular tissues, whereas verapamil primarily targets the heart muscle.
Diltiazem vs. Amlodipine: Diltiazem and amlodipine both cause vasodilation, but amlodipine has a longer half-life and primarily affects vascular smooth muscle, making it more suitable for long-term blood pressure control.
Diltiazem’s unique intermediate specificity and its ability to target both cardiac and vascular smooth muscle make it a versatile and effective therapeutic agent for various cardiovascular conditions.
生物活性
Diltiazem is primarily known as a calcium channel blocker used in the treatment of hypertension and certain cardiac conditions. However, emerging research has revealed its diverse biological activities beyond cardiovascular effects, including antimicrobial and antiviral properties. This article explores the multifaceted biological activity of Diltiazem, supported by recent studies and findings.
Diltiazem functions mainly by inhibiting L-type calcium channels in vascular smooth muscle and cardiac tissues, leading to vasodilation and reduced heart rate. This mechanism is crucial for its antihypertensive and antianginal effects. Additionally, studies suggest that Diltiazem may act as a pharmacological chaperone, enhancing the activity of certain enzymes in lysosomal storage diseases, such as Gaucher disease .
Antimicrobial Activity
Recent investigations have highlighted the antimicrobial properties of Diltiazem, particularly against Gram-positive bacteria. Notably:
- Inhibition of Biofilm Formation : Diltiazem hydrochloride significantly reduced biofilm formation in Staphylococcus aureus and Staphylococcus epidermidis by 90.7% and 95.1%, respectively, at sub-MIC concentrations .
- Antiviral Properties : The compound also exhibited antiviral activity against Coxsackievirus B4 (CoxB4), with an IC50 value of 35.8 μg/mL, demonstrating potential utility in viral infections .
Table 1: Antimicrobial Efficacy of Diltiazem Hydrochloride
| Bacteria | Biofilm Reduction (%) | IC50 (μg/mL) |
|---|---|---|
| Staphylococcus aureus | 90.7 | - |
| Staphylococcus epidermidis | 95.1 | - |
| Coxsackievirus B4 | - | 35.8 |
Cardiovascular Applications
Diltiazem is extensively studied for its role in managing coronary vasomotor dysfunction and atrial fibrillation:
- Coronary Vasomotor Dysfunction : A clinical trial showed that Diltiazem improved coronary flow reserve and reduced symptoms in patients with coronary vasospasm compared to placebo, indicating its effectiveness in managing microvascular dysfunction .
- Atrial Fibrillation : In a retrospective study involving patients with rapid atrial fibrillation, Diltiazem significantly reduced ventricular response rates (VRR) compared to controls, demonstrating its efficacy in restoring normal heart rhythm .
Table 2: Clinical Outcomes with Diltiazem Treatment
| Condition | Outcome Measure | Diltiazem Group (%) | Placebo Group (%) |
|---|---|---|---|
| Coronary Vasomotor Dysfunction | Improvement | 47 | 6 |
| Atrial Fibrillation | VRR Reduction to <100 bpm | 22.6 (OR) | - |
Case Studies
- Case Study on AV Block : A patient experiencing complete atrioventricular block due to coronary spasm was treated with intravenous Diltiazem, resulting in prompt relief of symptoms and restoration of sinus rhythm within minutes .
- Study on Microvascular Dysfunction : In a cohort study involving patients with coronary artery disease, those treated with Diltiazem showed significant improvement in coronary flow reserve after six weeks compared to those receiving placebo .
特性
IUPAC Name |
[(2S,3S)-5-[2-(dimethylamino)ethyl]-2-(4-methoxyphenyl)-4-oxo-2,3-dihydro-1,5-benzothiazepin-3-yl] acetate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C22H26N2O4S/c1-15(25)28-20-21(16-9-11-17(27-4)12-10-16)29-19-8-6-5-7-18(19)24(22(20)26)14-13-23(2)3/h5-12,20-21H,13-14H2,1-4H3/t20-,21+/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
HSUGRBWQSSZJOP-RTWAWAEBSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(=O)OC1C(SC2=CC=CC=C2N(C1=O)CCN(C)C)C3=CC=C(C=C3)OC | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC(=O)O[C@@H]1[C@@H](SC2=CC=CC=C2N(C1=O)CCN(C)C)C3=CC=C(C=C3)OC | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C22H26N2O4S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID9022940 | |
| Record name | (+)-Diltiazem | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID9022940 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
414.5 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Diltiazem | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014487 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Boiling Point |
Decomposes | |
| Record name | DILTIAZEM | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6528 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Solubility |
Soluble in methanol or chloroform, In water, 465 mg/l @ 25 °C, 1.68e-02 g/L | |
| Record name | Diltiazem | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00343 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | DILTIAZEM | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6528 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Diltiazem | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014487 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Excitation of cardiac muscle involves the activation of a slow calcium inward current that is induced by L-type slow calcium channels, which are voltage-sensitive, ion-selective channels associated with a high activation threshold and slow inactivation profile. L-type calcium channels are the main current responsible for the late phase of the pacemaker potential. Acting as the main Ca2+ source for contraction in smooth and cardiac muscle, activation of L-type calcium channels allows the influx of calcium ions into the muscles upon depolarization and excitation of the channel. It is proposed that this cation influx may also trigger the release of additional calcium ions from intracellular storage sites. Diltiazem is a slow calcium channel blocker that binds to the extracellular site of the alpha-1C subunit of the channel, which is thought to be the S5-6 linker region of the transmembrane domain IV and/or S6 segment of domain III. Diltiazem can get access to this binding site from either the intracellular or extracellular side, but it requires a voltage-induced conformational changes in the membrane. Diltiazem inhibits the influx of extracellular calcium across the myocardial and vascular smooth muscle cell membranes. In isolated human atrial and ventricular myocardium, diltiazem suppressed tension over the range of membrane potentials associated with calcium channel activity but had little effect on the tension-voltage relations at more positive potentials. This effect is thought to be mediated by the voltage-dependent block of the L-type calcium channels and inhibition of calcium ion release from the ER stores, without altering the sodium-calcium coupled transport or calcium sensitivity of myofilaments. Through inhibition of inward calcium current, diltiazem exerts a direct ionotropic and energy sparing effect on the myocardium. Diltiazem fslows atrioventricular nodal conduction, which is due to its ability to impede slow channel function. Reduced intracellular calcium concentrations equate to increased smooth muscle relaxation resulting in arterial vasodilation and therefore, decreased blood pressure. The decrease in intracellular calcium inhibits the contractile processes of the myocardial smooth muscle cells, causing dilation of the coronary and systemic arteries, increased oxygen delivery to the myocardial tissue, decreased total peripheral resistance, decreased systemic blood pressure, and decreased afterload. Through its actions on reducing calcium levels in cardiac and vascular smooth muscles, diltiazem causes a reduction in the contractile processes of the myocardial smooth muscle cells and vasodilation of the coronary and systemic arteries, including epicardial and subendocardial. This subsequently leads to increased oxygen delivery to the myocardial tissue, improved cardiac output due to increased stroke volume, decreased total peripheral resistance, decreased systemic blood pressure and heart rate, and decreased afterload. Diltiazem lowers myocardial oxygen demand through a reduction in heart rate, blood pressure, and cardiac contractility; this leads to a therapeutic effect in improving exercise tolerance in chronic stable angina., The effects of D-cis- and L-cis-diltiazem on the hydrogen peroxide (H2O2)-induced derangements of mechanical function and energy metab, and accumulation of intracellular Na+ were studied in isolated rat hearts. The intracellular concn of Na+ ([Na+]i) in the myocardium was measured using a nuclear magnetic resonance technique. H2O2 (600 uM) increased the left ventricular end-diastolic pressure, decreased the tissue level of ATP, and increased the release of lactate dehydrogenase from the myocardium. These alterations induced by H2O2 were significantly attenuated by D-cis-diltiazem (15 uM) or L-cis-diltiazem (15 uM). H2O2 (1 mM) produced a marked incr in the myocardial [Na+]i, which was effectively inhibited by ... D-cis-diltiazem (15 uM) or L-cis-diltiazem (15 uM). ... The protective action of D-cis- and L-cis-diltiazem may be due to their ability to inhibit the H2O2-induced incr in [Na+]i, at least in part. | |
| Record name | Diltiazem | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00343 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | DILTIAZEM | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6528 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
White crystalline powder | |
CAS No. |
56209-45-1, 42399-41-7 | |
| Record name | dl-cis-Diltiazem | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=56209-45-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Diltiazem | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=42399-41-7 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Diltiazem [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0042399417 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | cis-3-Acetoxy-5-(2-(dimethylamino)ethyl)-2,3-dihydro-2-(4-methoxyphenyl)-1,5-benzothiazepin-4(5H)-one | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0056209451 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Diltiazem | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00343 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | (+)-Diltiazem | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID9022940 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Diltiazem | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.050.707 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | DILTIAZEM | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/EE92BBP03H | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | DILTIAZEM | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6528 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Diltiazem | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014487 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
187-188, 212 °C (decomposes), 231 °C | |
| Record name | Diltiazem | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00343 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | DILTIAZEM | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/6528 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Diltiazem | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014487 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods
Procedure details










Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。














