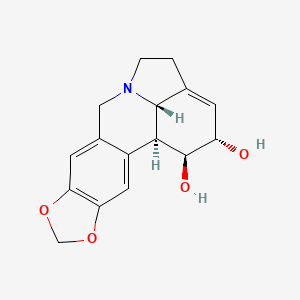
リコリン
概要
説明
科学的研究の応用
Lycorine is a naturally occurring alkaloid that has garnered attention for its diverse range of pharmacological activities, particularly in the realm of cancer research. Studies have explored its potential as an anti-cancer agent, demonstrating its effects on various cancer cell lines and tumor models .
Scientific Research Applications
Anti-Cancer Activity: Lycorine has demonstrated anticancer activities in several studies . It exhibits selective cytotoxicity effects on leukemia, cervical cancer, and multiple myeloma . Research indicates that lycorine can decrease proliferation, migration, invasion, and survival of prostate cancer cell lines . In vivo experiments have shown that lycorine reduces both the weight and volume of prostate cancer xenografts and inhibits cancer growth and metastasis in multiple organs, improving survival rates in mice .
Mechanism of Action in Cancer Cells: Lycorine's anti-cancer effects are linked to its ability to interfere with key signaling pathways involved in cancer development and progression . It inhibits the activation of EGF-induced JAK/STAT signaling and multiple STAT3 downstream targets, such as cyclin D1, Bcl-2, Bcl-xL, matrix metalloproteinase 2 (MMP2), and the EMT promoter Twist . In colorectal cancer cells, lycorine may induce cell cycle arrest and exert cytostatic effects by activating ROS/p38 and AKT signaling pathways . It can also downregulate the protein expression levels of MMP-2, MMP-7, and MMP-9, and reverse the EMT process in CRC cells .
Effects on Cell Cycle Arrest: Lycorine can induce cell cycle arrest in cancer cells . In HCT116 cells, lycorine treatment results in a significantly higher proportion of cells in the G2/M phase, while in LoVo cells, it leads to a significantly higher ratio of cells in the S and G2/M phases . Lycorine also downregulated the protein expression levels of cyclin D1 and cyclin E1, and increased p21 and Smad4 protein expression levels .
Other Pharmacological Effects: Lycorine possesses strong pharmacological effects on many diseases, including anti-leukemia, anti-tumor, anti-angiogenesis, anti-virus, and anti-bacteria . It has a strong anti-acetylcholinesterase effect .
Safety in the Central Nervous System: In vitro studies suggest that lycorine regulates immune cells, indicating its involvement as a cellular mechanism underlying the suppression of pain .
Tumor Xenograft Studies: Lycorine is effective against tumor xenografts and has effectively inhibited tumor growth in B16F10 melanoma-bearing mice, HL-60 xenografted SCID mice, ovarian cancer Hey1B bearing nude mice, and multiple myeloma (MM) cell xenografted NOD/SCID mice .
Tables
Table 1: Lycorine's Effects on Prostate Cancer Cell Lines (in vitro)
Table 2: Lycorine's Impact on Colorectal Cancer (CRC) Cell Lines (in vitro)
Case Studies
Prostate Cancer Xenograft Model: In vivo experiments using a PC-3M-luc orthotopic xenograft model demonstrated that lycorine inhibits prostate cancer growth and metastasis . Intraperitoneal administration of lycorine reduced both the weight and volume of ectopically PC-3M subcutaneous xenografts by approximately 80% without causing obvious toxicity .
Colorectal Cancer Cell Lines: Lycorine inhibited cell proliferation in HCT116, LoVo, and SW480 cells, with IC50 values of 1.4, 3.8, and 1.3 µmol/l, respectively, following treatment for 72 hours .
作用機序
生化学分析
Biochemical Properties
Lycorine interacts with various enzymes, proteins, and other biomolecules. It has been found to inhibit acetylcholinesterase and topoisomerase . The nature of these interactions contributes to its multiple biological functions and pharmacological effects .
Cellular Effects
Lycorine influences cell function by impacting cell signaling pathways, gene expression, and cellular metabolism . It has shown strong pharmacological effects on many diseases, including anti-leukemia, anti-tumor, anti-angiogenesis, anti-virus, anti-bacteria, anti-inflammation, and antimalaria .
Molecular Mechanism
Lycorine exerts its effects at the molecular level through binding interactions with biomolecules, enzyme inhibition or activation, and changes in gene expression . For instance, it has been found to have strong binding efficiency with key genes involved in glioblastoma multiforme (GBM), inducing apoptosis and reactive oxygen species production .
Temporal Effects in Laboratory Settings
Over time, lycorine has shown stability and long-term effects on cellular function in both in vitro and in vivo studies . It has been produced sustainably in in vitro culture due to the pharmaceutical industries dramatically increasing demand for it .
Dosage Effects in Animal Models
The effects of lycorine vary with different dosages in animal models . It exhibits numerous pharmacological effects on various diseases with very low toxicity and mild side effects .
Metabolic Pathways
Lycorine is involved in metabolic pathways formed by a coupling reaction of L-phenylalanine and L-tyrosine through intermediate o-methylnorbelladine—a common precursor of all Amaryllidaceae alkaloids .
準備方法
合成経路と反応条件
リコリンは、いくつかの方法で合成できます。 注目すべき合成経路の1つは、シクロプロピルアシルイミニウムイオンの拡張と転位、続いて重要なジエン中間体を構築するディールス・アルダー反応を用いる方法です . この方法は、Boeckmanらのグループによって開発され、リコリンの最もエレガントな合成経路の1つと考えられています .
工業生産方法
リコリンの工業生産は、通常、植物源からの抽出によって行われます。 シュテルンベルギア・フィシェリアナなどの植物の風乾された粉末球根を使用して、リコリンを単離します . 植物細胞懸濁培養やバイオリアクターなどの高度な技術も、リコリンをサステナビリティに生産するために用いられています .
化学反応の分析
反応の種類
リコリンは、酸化、還元、置換など、さまざまな化学反応を起こします。 リコリンはタンパク質合成を阻害することが知られており、アスコルビン酸の生合成を阻害する可能性もあります .
一般的な試薬と条件
リコリン反応で一般的に使用される試薬には、酸化反応のための酸化剤と、還元反応のための還元剤が含まれます。具体的な条件は、目的の反応と生成物によって異なります。
生成される主な生成物
リコリン反応から生成される主な生成物には、生物活性を高めた誘導体が含まれます。 たとえば、リコリンの水素化によって生成されるジヒドロリコリンは、アメーバ性赤痢に対する耐性が高く、毒性が低いことから、臨床的に使用されています .
科学研究への応用
リコリンは、さまざまな科学研究で応用されています。
類似化合物との比較
リコリンは、その剛性のある環状骨格と隣接するキラル中心のために、アルカロイドの中でも独特です . 類似の化合物には、以下のようなものがあります。
ガランタミン: アルツハイマー病の治療に使用されます。
リコラミン: ポリオ後遺症の治療に使用されます.
ジヒドロリコリン: 毒性が低く、アメーバ性赤痢に対する抵抗性が高いことから、臨床的に使用されています.
これらの化合物はリコリンと構造的な類似性を共有していますが、薬理学的特性と用途は異なります。
生物活性
Lycorine is a natural alkaloid derived from various plants, particularly those in the Amaryllidaceae family. It has garnered significant attention due to its diverse biological activities, including anti-cancer, antiviral, and neuroprotective properties. This article provides a comprehensive overview of the biological activity of lycorine, supported by research findings and data tables.
Lycorine's chemical structure is characterized by a benzylphenethylamine backbone, which is crucial for its biological activity. The compound exhibits multiple mechanisms of action, primarily through the inhibition of various cellular pathways associated with cancer and viral infections.
- Inhibition of DNA Topoisomerase I : Lycorine has been shown to inhibit DNA topoisomerase I, an enzyme critical for DNA replication in cancer cells. This inhibition leads to cytostatic effects rather than cytotoxicity, making it a potential candidate for cancer therapy .
- Autophagy-Associated Apoptosis : Research indicates that lycorine can induce autophagy-associated apoptosis by targeting MEK2, a key regulator in cancer signaling pathways. This mechanism enhances the therapeutic efficacy when combined with other agents like vemurafenib in colorectal cancer models .
- Cell Cycle Arrest : Lycorine has been found to induce cell cycle arrest in various cancer cell lines, specifically at the G2/M phase, thereby inhibiting cell proliferation and migration .
Anticancer Activity
Lycorine exhibits significant anticancer properties across various cancer types:
- Breast and Prostate Cancer : It promotes LRP6 phosphorylation and Wnt signaling transduction, which are beneficial against breast and prostate cancers .
- Bladder Cancer : In bladder cancer cell lines, lycorine enhances sensitivity to gemcitabine, a common chemotherapeutic agent .
- Leukemia : Studies have demonstrated that lycorine suppresses tumor growth in leukemia models (HL-60 cell line) through apoptosis induction .
Antiviral Activity
Lycorine has been identified as an effective inhibitor of Hepatitis C Virus (HCV), acting by downregulating Hsc70 expression in host cells. However, its cytotoxicity limits its clinical application as an antiviral agent .
Neuroprotective Effects
Lycorine exhibits analgesic properties superior to common analgesics like aspirin and indomethacin. It also shows anticonvulsant effects in animal models, indicating potential use in treating neurological disorders .
Table 1: Summary of Biological Activities of Lycorine
Case Studies
- Colorectal Cancer Study : A study demonstrated that lycorine treatment led to significant apoptosis in HCT116-derived xenografts, with enhanced effects observed when combined with vemurafenib .
- Bladder Cancer Sensitivity : In T24 and 5637 bladder cancer cells, lycorine increased gemcitabine sensitivity by approximately 40-60%, indicating its potential as an adjunct therapy .
- Cytotoxicity Studies : Structure-activity relationship studies revealed that modifications to lycorine's structure could reduce its cytotoxicity while maintaining antiviral efficacy against HCV .
特性
IUPAC Name |
(1S,17S,18S,19S)-5,7-dioxa-12-azapentacyclo[10.6.1.02,10.04,8.015,19]nonadeca-2,4(8),9,15-tetraene-17,18-diol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C16H17NO4/c18-11-3-8-1-2-17-6-9-4-12-13(21-7-20-12)5-10(9)14(15(8)17)16(11)19/h3-5,11,14-16,18-19H,1-2,6-7H2/t11-,14-,15+,16+/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
XGVJWXAYKUHDOO-DANNLKNASA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1CN2CC3=CC4=C(C=C3C5C2C1=CC(C5O)O)OCO4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C1CN2CC3=CC4=C(C=C3[C@H]5[C@H]2C1=C[C@@H]([C@H]5O)O)OCO4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C16H17NO4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID60197208 | |
| Record name | Lycorine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID60197208 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
287.31 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
476-28-8 | |
| Record name | Lycorine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=476-28-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Lycorine | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000476288 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | lycorine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=683873 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | lycorine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=401360 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Lycorine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID60197208 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Lycorine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.006.822 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | LYCORINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/I9Q105R5BU | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。















