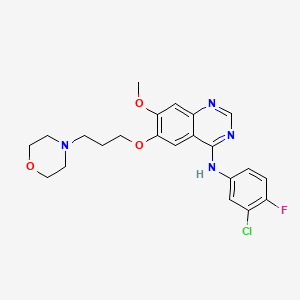
ゲフィチニブ
概要
説明
ゲフィチニブは、イレッサという商品名で販売されている薬剤であり、主に非小細胞肺がんや乳がんを含む特定の種類のがんの治療に使用されます。 これは、細胞の成長と生存の調節に重要な役割を果たす上皮成長因子受容体(EGFR)チロシンキナーゼの選択的阻害剤です .
2. 製法
合成経路および反応条件: ゲフィチニブは、4,5-ジメトキシ-2-ニトロ安息香酸から始まる多段階プロセスによって合成されます。 合成には、脱メチル化、エステル化、側鎖アルキル化、還元、シクロヘキシルアミン形成、塩素化、およびアンモニア置換など、いくつかの重要なステップが含まれます .
工業生産方法: 工業環境では、ゲフィチニブは、高収率と高純度を確保するために最適化された合成経路を使用して製造されています。 このプロセスには、目的の製品品質を実現するために、温度、pH、溶媒選択などの反応条件を厳密に制御することが含まれます .
科学的研究の応用
Non-Small Cell Lung Cancer (NSCLC)
- First-Line Treatment : Gefitinib is approved for use as a first-line treatment in patients with advanced NSCLC harboring sensitive EGFR mutations. Clinical trials have shown that gefitinib significantly improves progression-free survival compared to standard chemotherapy in this patient population .
- Second-Line Treatment : In cases where patients have previously received chemotherapy, gefitinib remains an effective option. Studies indicate that it offers better tolerability and quality of life compared to traditional chemotherapy regimens .
- Brain Metastases : One of the notable advantages of gefitinib is its ability to penetrate the blood-brain barrier effectively. This property makes it a viable option for treating patients with brain metastases from NSCLC, where other therapies may fail to achieve therapeutic concentrations .
Other Cancers
While gefitinib's primary application is in NSCLC, research is ongoing into its efficacy against other malignancies:
- Head and Neck Cancers : Some studies suggest potential benefits in head and neck squamous cell carcinoma, particularly in tumors expressing high levels of EGFR .
- Combination Therapies : Recent investigations have explored the synergistic effects of gefitinib when combined with other agents like anlotinib, showing enhanced efficacy against resistant NSCLC cell lines .
Case Study 1: Efficacy in Asian Populations
A significant body of research indicates that gefitinib is particularly effective among Asian populations with adenocarcinoma histology and those who have never smoked. The IRESSA Pan-Asia Study (IPASS) revealed an overall response rate exceeding 80% in patients with EGFR mutation-positive tumors . This study emphasizes the importance of genetic profiling in optimizing treatment strategies.
Case Study 2: Long-Term Outcomes
A longitudinal study involving patients treated with gefitinib over several years demonstrated sustained efficacy and manageable side effects. Patients reported improved quality of life metrics compared to those receiving standard chemotherapy regimens .
Adverse Effects
While gefitinib is generally well-tolerated, it can cause side effects such as skin rash, diarrhea, and liver enzyme elevation. Monitoring and management strategies are essential to mitigate these effects during treatment .
作用機序
ゲフィチニブは、EGFRチロシンキナーゼを阻害することにより効果を発揮します。それは酵素のアデノシン三リン酸(ATP)結合部位に結合し、下流シグナル伝達経路のリン酸化と活性化を阻止します。 この阻害は、EGFRが過剰発現または変異しているがん細胞における細胞増殖の抑制とアポトーシスの誘導につながります .
6. 類似の化合物との比較
ゲフィチニブは、エルロチニブやアファチニブなどの他のEGFR阻害剤と比較されることがよくあります。3つの化合物すべてがEGFRチロシンキナーゼを標的にしていますが、薬物動態特性と臨床的有効性に違いがあります。
エルロチニブ: 同様の作用機序ですが、副作用プロファイルと投与量レジメンが異なります。
独自性: ゲフィチニブは、EGFRチロシンキナーゼを選択的に阻害し、がん細胞の特定の変異を標的にできるという点でユニークであり、個別化がん療法において貴重なツールとなっています .
類似の化合物:
- エルロチニブ
- アファチニブ
- ラパチニブ
- オシメルチニブ
生化学分析
Biochemical Properties
Gefitinib plays a significant role in biochemical reactions. It interacts with various enzymes, proteins, and other biomolecules. Gefitinib is an inhibitor of the epidermal growth factor receptor (EGFR) tyrosine kinase that binds to the adenosine triphosphate (ATP)-binding site of the enzyme . EGFR is often overexpressed in certain human carcinoma cells, such as lung and breast cancer cells .
Cellular Effects
Gefitinib has profound effects on various types of cells and cellular processes. It influences cell function, including impacts on cell signaling pathways, gene expression, and cellular metabolism. In vitro cytotoxicity studies revealed that Gefitinib enhanced the inhibition of cell proliferation and apoptosis in A549 and H1299 cells compared to free Gefitinib .
Molecular Mechanism
Gefitinib exerts its effects at the molecular level through several mechanisms. It binds to the ATP-binding site of the EGFR tyrosine kinase enzyme, inhibiting its activity . This interaction leads to changes in gene expression and cellular functions .
準備方法
Synthetic Routes and Reaction Conditions: Gefitinib is synthesized through a multi-step process starting from 4,5-dimethoxy-2-nitrobenzoic acid. The synthesis involves several key steps, including demethylation, esterification, side-chain alkylation, reduction, cyclohexylamine formation, chlorination, and ammonia substitution .
Industrial Production Methods: In industrial settings, gefitinib is produced using optimized synthetic routes to ensure high yield and purity. The process involves strict control of reaction conditions, such as temperature, pH, and solvent selection, to achieve the desired product quality .
化学反応の分析
反応の種類: ゲフィチニブは、以下を含むさまざまな化学反応を受けます。
酸化: ゲフィチニブは、さまざまな代謝産物を生成するために酸化することができます。
還元: 還元反応は、キナゾリン環構造を変更することができます。
一般的な試薬と条件:
酸化: 一般的な酸化剤には、過酸化水素と過マンガン酸カリウムがあります。
還元: 水素化ホウ素ナトリウムや水素化リチウムアルミニウムなどの還元剤が使用されます。
主要な生成物: これらの反応から形成される主要な生成物には、薬理学的性質が変化したさまざまなゲフィチニブ誘導体が含まれます .
4. 科学研究の応用
ゲフィチニブは、幅広い科学研究の応用を持っています。
化学: EGFR阻害剤とその標的タンパク質との相互作用を研究するためのモデル化合物として使用されています。
生物学: 細胞シグナル伝達経路とアポトーシスへの影響について調査されています。
医学: 非小細胞肺がんやその他のEGFR変異がんの治療のための臨床試験で広く使用されています。
類似化合物との比較
- Erlotinib
- Afatinib
- Lapatinib
- Osimertinib
生物活性
Gefitinib, an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, is primarily used in the treatment of non-small cell lung cancer (NSCLC) with specific EGFR mutations. This article explores the biological activity of gefitinib, focusing on its mechanisms of action, efficacy in clinical trials, and emerging research findings.
Gefitinib selectively binds to the ATP-binding site of the EGFR tyrosine kinase domain, inhibiting its phosphorylation and subsequent activation of downstream signaling pathways. This results in decreased cell proliferation and increased apoptosis in cancer cells expressing mutant forms of EGFR. The compound has shown significant effects on various cellular processes, including:
- Inhibition of Cell Proliferation : Gefitinib suppresses the growth of cancer cells by blocking EGFR-mediated signaling pathways.
- Induction of Apoptosis : It promotes programmed cell death through alterations in mitochondrial function and expression levels of Bcl-2 family proteins, such as Bcl-xL and Bax .
- Impact on Mitochondrial Activity : Recent studies indicate that gefitinib enhances mitochondrial functions, such as succinate-tetrazolium reductase (STR) activity, particularly in high-density cell cultures .
Efficacy in Clinical Trials
Gefitinib has been evaluated extensively in clinical trials for its efficacy and safety profile. Key findings from several studies are summarized below:
Case Studies
- Mitochondrial Activity Enhancement : In a study involving HCC827 cells (EGFR mutation positive), gefitinib was shown to enhance mitochondrial membrane potential and STR activity, indicating its role as a mitochondrial protector during combination therapy with doxorubicin .
- Resistance Development : Despite its initial effectiveness, resistance to gefitinib often develops within one to two years due to various mechanisms including secondary mutations in the EGFR gene or activation of alternative signaling pathways . A case study highlighted a patient who initially responded well but later exhibited resistance due to a T790M mutation.
- Urothelial Carcinoma : In vitro studies demonstrated gefitinib's inhibitory effects on growth and invasion in urothelial carcinoma cell lines, suggesting potential applications beyond NSCLC .
特性
IUPAC Name |
N-(3-chloro-4-fluorophenyl)-7-methoxy-6-(3-morpholin-4-ylpropoxy)quinazolin-4-amine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
XGALLCVXEZPNRQ-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
COC1=C(C=C2C(=C1)N=CN=C2NC3=CC(=C(C=C3)F)Cl)OCCCN4CCOCC4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C22H24ClFN4O3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID8041034 | |
| Record name | Gefitinib | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8041034 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
446.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Gefitinib | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014462 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
Sparingly soluble (|
|
|
| Record name | Gefitinib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00317 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Gefitinib | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014462 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Gefitinib is an inhibitor of the epidermal growth factor receptor (EGFR) tyrosine kinase that binds to the adenosine triphosphate (ATP)-binding site of the enzyme. EGFR is often shown to be overexpressed in certain human carcinoma cells, such as lung and breast cancer cells. Overexpression leads to enhanced activation of the anti-apoptotic Ras signal transduction cascades, subsequently resulting in increased survival of cancer cells and uncontrolled cell proliferation. Gefitinib is the first selective inhibitor of the EGFR tyrosine kinase which is also referred to as Her1 or ErbB-1. By inhibiting EGFR tyrosine kinase, the downstream signaling cascades are also inhibited, resulting in inhibited malignant cell proliferation. | |
| Record name | Gefitinib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00317 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
184475-35-2 | |
| Record name | Gefitinib | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=184475-35-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Gefitinib [USAN:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0184475352 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Gefitinib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00317 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Gefitinib | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=759856 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Gefitinib | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID8041034 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Gefitinib | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | GEFITINIB | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/S65743JHBS | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Gefitinib | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014462 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details







Synthesis routes and methods II
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。














