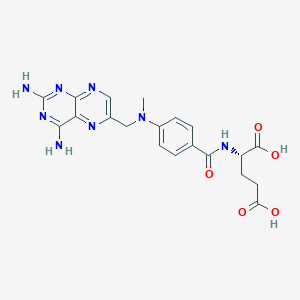
Methotrexate
概要
説明
メトトレキセートは、以前はアメトプテリンとして知られており、化学療法薬および免疫抑制剤です。様々な種類の癌、自己免疫疾患、および異所性妊娠の治療に広く使用されています。 この化合物は、特に乳がん、白血病、肺がん、リンパ腫、妊娠性絨毛性疾患、骨肉腫に効果的です . さらに、乾癬、関節リウマチ、クローン病などの自己免疫疾患の管理にも使用されます .
2. 製法
合成ルートおよび反応条件: メトトレキセートは、4-アミノ安息香酸と2,4-ジアミノ-6-ヒドロキシピリミジンを反応させて4-アミノ-4-デオキシ-N10-メチルプテロイル酸を生成する多段階プロセスによって合成されます。 この中間体は、次にL-グルタミン酸と結合してメトトレキセートを生成します .
工業生産方法: メトトレキセートの工業生産には、実験室合成と同様の反応条件を使用して、収率と純度を向上させた大規模合成が含まれます。 このプロセスには、結晶化やクロマトグラフィーなどの厳格な精製工程が含まれ、最終製品が医薬品基準を満たしていることを保証します .
準備方法
Synthetic Routes and Reaction Conditions: Methotrexate is synthesized through a multi-step process involving the reaction of 4-aminobenzoic acid with 2,4-diamino-6-hydroxypyrimidine to form 4-amino-4-deoxy-N10-methylpteroic acid. This intermediate is then coupled with L-glutamic acid to produce this compound .
Industrial Production Methods: Industrial production of this compound involves large-scale synthesis using similar reaction conditions as the laboratory synthesis but optimized for higher yields and purity. The process includes rigorous purification steps such as crystallization and chromatography to ensure the final product meets pharmaceutical standards .
化学反応の分析
Enzymatic Interactions and Binding Mechanisms
MTX exerts its effects through tight binding to folate-dependent enzymes, mediated by structural rearrangements:
Dihydrofolate Reductase (DHFR)
- Binding affinity : due to hydrogen bonds between MTX’s N2/N4 and DHFR’s Glu30/backbone .
- Inhibition : Competes with dihydrofolate () .
Serine Hydroxymethyltransferases (SHMTs)
| Enzyme | IC₅₀ (MTX) | Key Residues Involved |
|---|---|---|
| SHMT2 | 4.8 µM | Glu104*, Lys414, Thr416 |
| SHMT4 | 3.2 µM | Ala423, Glu104* |
Metabolic Pathways and Biotransformations
MTX undergoes intracellular polyglutamation and oxidation, altering its pharmacokinetics and toxicity profile:
- Key metabolites :
Stability and Degradation Reactions
MTX degrades under specific conditions, impacting its storage and administration:
Allosteric Modulation of Protein Complexes
MTX induces conformational changes in proteins to enable novel interactions:
科学的研究の応用
Oncological Applications
Methotrexate is a cornerstone in the treatment of various malignancies due to its ability to inhibit DNA synthesis by acting as a folate antagonist. Its applications include:
- Acute Lymphoblastic Leukemia : this compound is a first-line treatment, particularly in pediatric cases, often used in combination with other chemotherapeutic agents.
- Breast Cancer : It is utilized in combination regimens for advanced stages.
- Non-Hodgkin Lymphoma : Effective in relapsed or refractory cases, this compound is part of multi-agent chemotherapy protocols.
- Gestational Trophoblastic Neoplasia : Used to treat this rare condition effectively.
| Cancer Type | Indication |
|---|---|
| Acute Lymphoblastic Leukemia | First-line treatment |
| Breast Cancer | Advanced stages |
| Non-Hodgkin Lymphoma | Relapsed or refractory |
| Gestational Trophoblastic Neoplasia | Effective treatment |
Rheumatological Applications
This compound is widely employed as a disease-modifying antirheumatic drug (DMARD) for autoimmune diseases:
- Rheumatoid Arthritis : It significantly reduces disease activity and joint damage.
- Juvenile Idiopathic Arthritis : Approved for pediatric patients.
- Psoriasis : Effective for severe cases, particularly when systemic therapy is required.
| Condition | Efficacy |
|---|---|
| Rheumatoid Arthritis | High potency and efficacy |
| Juvenile Idiopathic Arthritis | Approved for pediatric use |
| Psoriasis | First-line systemic therapy |
Dermatological Applications
In dermatology, this compound is utilized for several chronic skin conditions:
- Psoriasis : this compound is considered a first-line systemic therapy.
- Atopic Dermatitis : Demonstrates efficacy, particularly in severe cases resistant to other treatments.
Case Study: this compound in Allergic Contact Dermatitis
A retrospective study involving 32 patients treated with this compound for allergic contact dermatitis showed that 78% experienced partial or complete responses. Notably, the efficacy was similar among patients with persistent allergen exposure, indicating its robustness as a treatment option .
Other Medical Applications
This compound's immunomodulatory properties extend its use to various other conditions:
- Ocular Inflammation : Effective in managing noninfectious ocular inflammatory diseases, achieving sustained control of inflammation in many patients.
- Inflammatory Bowel Disease : Utilized as an adjunctive therapy for ulcerative colitis and Crohn's disease.
Table: Summary of Non-Oncological Uses
| Condition | Application |
|---|---|
| Ocular Inflammation | Control of inflammation |
| Inflammatory Bowel Disease | Adjunctive therapy |
| Systemic Lupus Erythematosus | Effective treatment |
Toxicity and Management
While this compound is effective, it carries risks of toxicity, including:
- Bone marrow suppression
- Hepatotoxicity
- Pulmonary toxicity
Management strategies include monitoring renal function and using leucovorin as an antidote to mitigate toxicity effects .
作用機序
メトトレキセートは、ジヒドロ葉酸レダクターゼ、チミジル酸シンターゼ、アミノイミダゾールカルボキサミドリボヌクレオチドトランスホルミラーゼなど、ヌクレオチド合成に関与するいくつかの酵素を阻害することで効果を発揮します . この阻害は、DNA合成と細胞分裂の抑制につながり、急速に分裂する癌細胞に対して効果的です . さらに、メトトレキセートはアデノシンの放出を促進し、これは抗炎症効果があります .
6. 類似の化合物との比較
メトトレキセートは、葉酸とアミノプテリンと構造的に類似しています。 N-メチル化された形態は独特で、抗代謝物としての効力を高めています . 類似の化合物には以下が含まれます:
アミノプテリン: 以前の抗葉酸剤であり、メカニズムは似ていますが、毒性がより高くなっています.
ペメトレキセド: 癌治療に使用される別の抗葉酸剤であり、より広範囲な活性を示します.
ラルトレキセド: 大腸癌に使用されるチミジル酸シンターゼ阻害剤.
メトトレキセートの独自の構造と作用機序により、医学および研究においてさまざまな用途を持つ貴重な治療薬となっています。
類似化合物との比較
Methotrexate is structurally similar to folic acid and aminopterin. it is unique in its N-methylated form, which enhances its potency as an antimetabolite . Similar compounds include:
Aminopterin: An earlier antifolate agent with similar mechanisms but higher toxicity.
Pemetrexed: Another antifolate used in cancer treatment, with a broader spectrum of activity.
Raltitrexed: A thymidylate synthase inhibitor used in colorectal cancer.
This compound’s unique structure and mechanism of action make it a valuable therapeutic agent with diverse applications in medicine and research.
生物活性
Methotrexate (MTX) is a widely used anti-metabolite and immunosuppressant, primarily recognized for its role in treating various cancers and autoimmune diseases such as rheumatoid arthritis (RA). Its biological activity is characterized by a complex mechanism that affects cellular processes, particularly those involved in nucleotide synthesis and immune modulation.
This compound exerts its effects through several key mechanisms:
-
Inhibition of Dihydrofolate Reductase (DHFR) :
- MTX is taken up into cells via the human reduced folate carrier (SLC19A1), where it is converted into this compound-polyglutamate. This compound inhibits DHFR, blocking the conversion of dihydrofolate to tetrahydrofolate, a critical cofactor in nucleotide synthesis necessary for DNA and RNA production .
- Impact on Purine Synthesis :
- Immune Modulation :
Pharmacokinetics
- Absorption : this compound has a bioavailability ranging from 64% to 90%, although this decreases at doses above 25 mg due to saturation of transport mechanisms .
- Distribution : It is highly protein-bound, which can affect its clearance when co-administered with other drugs that displace it from plasma proteins .
- Elimination : The drug is primarily eliminated by renal excretion, making renal function a critical factor in its dosing and potential toxicity.
Case Studies and Research Findings
- Rheumatoid Arthritis :
- Long-term Safety :
-
Comparative Studies :
- In a double-blind study comparing MTX with azathioprine, MTX demonstrated superior efficacy in reducing disease activity and less radiographic progression over 48 weeks . Another study highlighted that MTX was better tolerated than intramuscular gold therapy, reinforcing its status as a first-line treatment for RA .
Adverse Effects
While this compound is effective, it carries risks of toxicity:
- Hepatotoxicity : Elevated liver enzymes have been reported, with some cases leading to permanent discontinuation of the drug due to liver damage .
- Nephrotoxicity : High doses can lead to acute kidney injury, particularly in patients with compromised renal function .
- Iatrogenic Toxicity : A case study illustrated severe toxicity due to unintentional overdose, emphasizing the need for careful dosing and monitoring .
Summary Table of Key Findings
| Study/Case | Findings | Implications |
|---|---|---|
| Long-term RA study | Significant reduction in joint symptoms over 36 months | Supports continued use as an effective long-term therapy |
| Meta-analysis on safety | No increased risk for CVD; liver enzyme elevation in 13% | Regular monitoring essential for long-term users |
| Comparative study with azathioprine | MTX superior in efficacy and safety profile | Reinforces MTX as first-line therapy |
特性
IUPAC Name |
(2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methylamino]benzoyl]amino]pentanedioic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
FBOZXECLQNJBKD-ZDUSSCGKSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN(CC1=CN=C2C(=N1)C(=NC(=N2)N)N)C3=CC=C(C=C3)C(=O)NC(CCC(=O)O)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CN(CC1=CN=C2C(=N1)C(=NC(=N2)N)N)C3=CC=C(C=C3)C(=O)N[C@@H](CCC(=O)O)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C20H22N8O5 | |
| Record name | METHOTREXATE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20602 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
15475-56-6 (hydrochloride salt), 7413-34-5 (di-hydrochloride salt) | |
| Record name | Methotrexate [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000059052 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID4020822 | |
| Record name | Methotrexate | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4020822 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
454.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Methotrexate is an odorless yellow to orange-brown crystalline powder. (NTP, 1992) It is a chemotherapy drug that interferes with DNA and RNA synthesis., Solid, Bright yellow-orange, odorless, crystalline powder. | |
| Record name | METHOTREXATE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20602 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Methotrexate | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014703 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
| Record name | METHOTREXATE | |
| Source | Occupational Safety and Health Administration (OSHA) | |
| URL | https://www.osha.gov/chemicaldata/874 | |
| Description | The OSHA Occupational Chemical Database contains over 800 entries with information such as physical properties, exposure guidelines, etc. | |
| Explanation | Materials created by the federal government are generally part of the public domain and may be used, reproduced and distributed without permission. Therefore, content on this website which is in the public domain may be used without the prior permission of the U.S. Department of Labor (DOL). Warning: Some content - including both images and text - may be the copyrighted property of others and used by the DOL under a license. | |
Solubility |
less than 1 mg/mL at 66 °F (NTP, 1992), Insoluble in water , alcohol, chloroform, ether; slightly soluble in dilute hydrochloric acid; soluble in dil solns of alkali hydroxides and carbonates., Practically insoluble in water and in alcohol., In water, 2.6X10+3 mg/L at 25 °C /Estimated/, 1.71e-01 g/L | |
| Record name | METHOTREXATE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20602 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | METHOTREXATE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3123 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Methotrexate | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014703 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Vapor Pressure |
2.1X10-19 mm Hg at 25 °C /Estimated/ | |
| Record name | METHOTREXATE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3123 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Mechanism of Action |
Methotrexate enters tissues and is converted to a methotrexate polyglutamate by folylpolyglutamate. Methotrexate's mechanism of action is due to its inhibition of enzymes responsible for nucleotide synthesis including dihydrofolate reductase, thymidylate synthase, aminoimidazole caboxamide ribonucleotide transformylase (AICART), and amido phosphoribosyltransferase. Inhibtion of nucleotide synthesis prevents cell division. In rheumatoid arthritis, methotrexate polyglutamates inhibit AICART more than methotrexate. This inhibition leads to accumulation of AICART ribonucleotide, which inhibits adenosine deaminase, leading to an accumulation of adenosine triphosphate and adenosine in the extracellular space, stimulating adenosine receptors, leading to anti-inflammatory action., Methotrexate and its polyglutanate metabolites reversibly inhibits dihydrofolate reductase, the enzyme that reduces folic acid to tetrahydrofolic acid. Inhibition of tetrahydrofolate formation limits the availability of one-carbon fragments necessary for synthesis of purines and the conversion of deoxyuridylate to thymidylate in the synthesis of DNA and cell reproduction. The affinity of dihydrofolate reductase for methotrexate is far greater than its affinity for folic acid or dihydrofolic acid. and, therefore, even very large doses of folic acid given simultaneously will not reverse the effects of methotrexate. Leucovorin calcium, a derivative of tetrahydrofolic acid, may block the effects of methotrexate if given shortly after the antineoplastic agent. Results of one study indicate that methotrexate also causes an increase in intracellular deoxyadenosine triphosphate, which is thought to inhibit ribonucleotide reduction, and polynucleotide ligase, an enzyme concerned in DNA synthesis and repair. Tissues with high rates of cellular proliferation such as neoplasms, psoriatic epidermis, bone marrow, the lining of the GI tract, hair matrix, and fetal cells are most sensitive to the effects of methotrexate., Methotrexate ... has immunosuppressive activity, in part possibly as a result of inhibition of lymphocyte multiplication. The mechanism(s) of action in the management of rheumatoid arthritis of the drug is not known, although suggested mechanisms have included immunosuppressive and/or antiinflammatory effects. | |
| Record name | Methotrexate | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00563 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | METHOTREXATE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3123 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Orange-brown, crystalline powder | |
CAS No. |
59-05-2 | |
| Record name | METHOTREXATE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20602 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Methotrexate | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=59-05-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Methotrexate [USAN:USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000059052 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Methotrexate | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00563 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | methotrexate | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=740 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | L-Glutamic acid, N-[4-[[(2,4-diamino-6-pteridinyl)methyl]methylamino]benzoyl]- | |
| Source | EPA Chemicals under the TSCA | |
| URL | https://www.epa.gov/chemicals-under-tsca | |
| Description | EPA Chemicals under the Toxic Substances Control Act (TSCA) collection contains information on chemicals and their regulations under TSCA, including non-confidential content from the TSCA Chemical Substance Inventory and Chemical Data Reporting. | |
| Record name | Methotrexate | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID4020822 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Methotrexate | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.376 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | METHOTREXATE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/YL5FZ2Y5U1 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | METHOTREXATE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3123 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Methotrexate | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014703 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
| Record name | METHOTREXATE | |
| Source | Occupational Safety and Health Administration (OSHA) | |
| URL | https://www.osha.gov/chemicaldata/874 | |
| Description | The OSHA Occupational Chemical Database contains over 800 entries with information such as physical properties, exposure guidelines, etc. | |
| Explanation | Materials created by the federal government are generally part of the public domain and may be used, reproduced and distributed without permission. Therefore, content on this website which is in the public domain may be used without the prior permission of the U.S. Department of Labor (DOL). Warning: Some content - including both images and text - may be the copyrighted property of others and used by the DOL under a license. | |
Melting Point |
365 to 399 °F (decomposes) (NTP, 1992), Melting point: 185-204 °C. /Methotrexate monohydrate/, 195 °C, 356-399 °F | |
| Record name | METHOTREXATE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20602 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Methotrexate | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00563 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | METHOTREXATE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3123 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Methotrexate | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014703 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
| Record name | METHOTREXATE | |
| Source | Occupational Safety and Health Administration (OSHA) | |
| URL | https://www.osha.gov/chemicaldata/874 | |
| Description | The OSHA Occupational Chemical Database contains over 800 entries with information such as physical properties, exposure guidelines, etc. | |
| Explanation | Materials created by the federal government are generally part of the public domain and may be used, reproduced and distributed without permission. Therefore, content on this website which is in the public domain may be used without the prior permission of the U.S. Department of Labor (DOL). Warning: Some content - including both images and text - may be the copyrighted property of others and used by the DOL under a license. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。
















