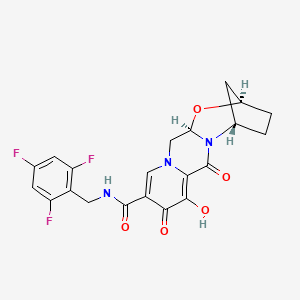
ビクテグラビル
概要
説明
ビクテグラビルは、ギリアド・サイエンシズが開発した、ヒト免疫不全ウイルス(HIV)感染症の治療のための第二世代インテグラーゼ鎖転移阻害剤です。これは、以前の化合物であるドルテグラビルから構造的に派生しています。 ビクテグラビルは、テノフォビルアラフェナミドやエムトリシタビンなどの他の抗レトロウイルス剤と組み合わせて使用され、HIV-1感染症の治療のための単剤療法となります .
科学的研究の応用
Efficacy in Clinical Trials
Phase 3 Studies
Bictegravir has been evaluated in several Phase 3 clinical trials. In these studies, it demonstrated non-inferiority compared to other standard regimens, such as dolutegravir combined with emtricitabine and tenofovir alafenamide. For instance, one study reported that after 96 weeks, 84% of participants on bictegravir achieved an HIV-1 RNA level of less than 50 copies/mL, comparable to 86% in the dolutegravir group .
Long-Term Effectiveness
The BICSTaR (BICtegravir Single Tablet Regimen) study provides real-world evidence supporting the long-term effectiveness of bictegravir. Data from this observational cohort study showed that after 12 months of treatment, HIV-1 RNA was suppressed to less than 50 copies/mL in 94% of treatment-naïve participants and 97% of treatment-experienced participants .
Safety Profile
Bictegravir's safety profile has been extensively documented. In clinical trials, drug-related adverse events were reported in approximately 20% of participants receiving bictegravir, which is lower compared to the dolutegravir group where adverse events were reported in about 28% . The most common side effects included gastrointestinal issues and headache, with serious adverse events being rare .
Real-World Applications
Diverse Populations
Research indicates that bictegravir is effective across diverse populations. A study focusing on Asian cohorts found that after 12 months of treatment with bictegravir/emtricitabine/tenofovir alafenamide, 98.2% of treatment-naïve participants achieved viral suppression . This highlights bictegravir's applicability in various demographic groups.
Combination Therapies
Bictegravir is also being explored in combination with other antiretroviral therapies for enhanced efficacy. For example, ongoing research into nanoparticle formulations combining bictegravir with tenofovir alafenamide aims to develop long-acting formulations that could improve adherence and outcomes in HIV prevention strategies .
Case Studies
Case Study: BICSTaR Study
In the BICSTaR study involving over 1500 participants from multiple countries, researchers assessed the effectiveness and safety of bictegravir/emtricitabine/tenofovir alafenamide. The results confirmed high rates of viral suppression and favorable tolerability over a year-long follow-up period .
Case Study: Long-Acting Formulations
A novel approach using a nanoformulation combining bictegravir with tenofovir alafenamide has shown promise in preclinical studies for long-acting HIV prevention. This formulation aims to provide sustained drug release, potentially reducing the frequency of dosing required for effective prophylaxis against HIV .
作用機序
ビクテグラビルは、HIV-1インテグラーゼ酵素を標的にすることで、ウイルスDNAのヒトゲノムへの鎖転移を阻害します。 これは、HIV-1ウイルスの宿主DNAへの組み込みを防ぎ、それによってウイルスの複製と増殖を阻止します . ビクテグラビルは、インビトロでHIV-1およびHIV-2のさまざまなサブタイプに対して強力な抗ウイルス活性を示しています .
生化学分析
Biochemical Properties
Bictegravir plays a crucial role in biochemical reactions as it inhibits the HIV-1 integrase enzyme . This enzyme is responsible for the transfer of virally encoded DNA into the host genome, a critical step in the HIV replication cycle . By inhibiting this process, Bictegravir prevents HIV-1 replication and propagation .
Cellular Effects
Bictegravir has a profound impact on various types of cells and cellular processes. It influences cell function by inhibiting HIV-1 virus replication into the human genome . This inhibition disrupts the viral life cycle and prevents the infection of new cells .
Molecular Mechanism
Bictegravir exerts its effects at the molecular level primarily through its interaction with the HIV-1 integrase enzyme . It inhibits the strand transfer of viral DNA into the host genome, thereby preventing HIV-1 replication .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of Bictegravir have been observed over time. For instance, in a study involving pregnant mice, Bictegravir was administered orally from gestational day 11.5 to 15.5, and drug concentrations in the maternal plasma were determined at 1 h and 24 h post drug administration .
Dosage Effects in Animal Models
The effects of Bictegravir vary with different dosages in animal models. In a pharmacokinetic dose-optimization study of Bictegravir in a mouse pregnancy model, pregnant mice were administered increasing doses of Bictegravir in combination with emtricitabine and tenofovir .
Metabolic Pathways
Bictegravir is metabolized in the liver and kidneys . It undergoes metabolism primarily through the liver (CYP3A4), so inducers of CYP3A4 should be avoided .
準備方法
化学反応の分析
ビクテグラビルは、酸化、還元、置換などのさまざまな化学反応を受けます。これらの反応で一般的に使用される試薬には、酸化剤、還元剤、求核剤などがあります。 これらの反応で生成される主な生成物は、使用される特定の条件と試薬によって異なります .
科学研究への応用
ビクテグラビルは、主にHIV-1感染症の治療のための医学分野で使用されています。 これは、テノフォビルアラフェナミドとエムトリシタビンとの配合剤であり、治療未経験の患者とウイルス抑制中の患者において有効性が示されています . ビクテグラビルは、現実世界の環境における有効性、安全性、および中止率について研究されています . さらに、HIV-2感染症の治療における可能性を評価するために、臨床試験で使用されています .
類似化合物との比較
生物活性
Bictegravir (BIC) is an integrase strand transfer inhibitor (INSTI) utilized in the treatment of HIV-1 infection. It is part of the combination therapy Biktarvy, which includes emtricitabine (FTC) and tenofovir alafenamide (TAF). BIC exhibits high potency and selectivity against HIV-1, demonstrating a favorable pharmacokinetic profile and resistance characteristics compared to other INSTIs like raltegravir (RAL) and elvitegravir (EVG) .
Bictegravir functions by inhibiting the HIV integrase enzyme, which is crucial for the viral replication cycle. Specifically, BIC binds to the integrase active site, blocking the strand transfer step necessary for viral DNA integration into the host genome. This action effectively halts viral replication and contributes to viral load reduction in patients .
Key Features of Bictegravir's Mechanism:
- High Potency : BIC shows superior antiviral activity against multiple HIV-1 variants.
- Resistance Profile : It has a longer dissociation half-life from integrase/DNA complexes than RAL and EVG, making it less susceptible to resistance mutations .
- Synergistic Activity : In vitro studies indicate that BIC, when combined with FTC and TAF, exhibits synergistic anti-HIV activity without antagonism .
Pharmacokinetics
Bictegravir is characterized by its favorable pharmacokinetic properties:
- Bioavailability : High oral bioavailability allows for once-daily dosing.
- Half-life : Extended half-life supports sustained antiviral effects.
- Drug Interactions : BIC is a substrate for P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP), necessitating caution when co-administered with drugs that inhibit these transporters .
Table 1: Pharmacokinetic Parameters of Bictegravir
| Parameter | Value |
|---|---|
| Bioavailability | High |
| Half-life | Approximately 18 hours |
| Protein Binding | ~90% |
| Metabolism | Minimal hepatic metabolism; primarily renal excretion |
Clinical Efficacy
Clinical trials have demonstrated that bictegravir-containing regimens are highly effective in achieving viral suppression in both treatment-naïve and treatment-experienced patients.
Case Studies
- Long-term Efficacy in Women and Older Adults :
- Nanoencapsulation Study :
Safety Profile
The safety profile of bictegravir is generally favorable, with no significant overlapping toxicities observed when used in combination with FTC and TAF. Studies indicate no evidence of genotoxicity or carcinogenicity associated with bictegravir, further supporting its clinical use .
Common Adverse Effects:
- Gastrointestinal disturbances
- Fatigue
- Headache
特性
IUPAC Name |
(1S,11R,13R)-5-hydroxy-3,6-dioxo-N-[(2,4,6-trifluorophenyl)methyl]-12-oxa-2,9-diazatetracyclo[11.2.1.02,11.04,9]hexadeca-4,7-diene-7-carboxamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C21H18F3N3O5/c22-9-3-14(23)12(15(24)4-9)6-25-20(30)13-7-26-8-16-27(10-1-2-11(5-10)32-16)21(31)17(26)19(29)18(13)28/h3-4,7,10-11,16,29H,1-2,5-6,8H2,(H,25,30)/t10-,11+,16+/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
SOLUWJRYJLAZCX-LYOVBCGYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1CC2CC1N3C(O2)CN4C=C(C(=O)C(=C4C3=O)O)C(=O)NCC5=C(C=C(C=C5F)F)F | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C1C[C@@H]2C[C@H]1N3[C@H](O2)CN4C=C(C(=O)C(=C4C3=O)O)C(=O)NCC5=C(C=C(C=C5F)F)F | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C21H18F3N3O5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID701027937 | |
| Record name | Bictegravir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID701027937 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
449.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Boiling Point |
682.5±55.0 °C at 760 mmHg | |
| Record name | Bictegravir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB11799 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Mechanism of Action |
This single dose medication inhibits the strand transfer of viral DNA into the human genome, preventing HIV-1 virus replication and propagation. In vitro, bictegravir has shown powerful antiviral activity against HIV-2 and various subtypes of HIV-1. It has shown synergistic effects when combined with other ARVs, including tenofovir alafenamide (TAF), emtricitabine (FTC), and darunavir (DRV). The three components of the first USA approved medication ( trade name: Biktarvy ) are as follows: Bictegravir: integrase strand transfer inhibitor; INSTI), an HIV-1 encoded enzyme necessary for viral replication. Inhibition of the integrase enzyme prevents the integration of HIV-1 into host DNA, blocking the conversion of the HIV-1 provirus and progression of the virus [FDA LABEL]. Emtricitabine: FTC, is phosphorylated by cellular enzymes to form emtricitabine 5'-triphosphate. Emtricitabine is phosphorylated to form emtricitabine 5'-triphosphate intracellularly. This metabolite inhibits the activity of human immunodeficiency virus (HIV) reverse transcriptase by competing with the substrate deoxycytidine 5'-triphosphate and by incorporating itself into viral DNA preventing DNA chain elongation [FDA LABEL]. Tenofovir Alafenamide: TAF is a phosphonamidate prodrug of tenofovir (2′-deoxyadenosine monophosphate analog). Plasma exposure to TAF leads to leakage into cells and then TAF is intracellularly converted to tenofovir by hydrolysis by cathepsin. Tenofovir is subsequently phosphorylated by cellular kinases to the metabolite tenofovir diphosphate, which is the active form of the drug. Tenofovir diphosphate inhibits HIV-1 replication by incorporating into viral DNA by the HIV reverse transcriptase, resulting in DNA chain-termination. Tenofovir diphosphate also weakly inhibits mammalian DNA polymerases [FDA LABEL]. | |
| Record name | Bictegravir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB11799 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
1611493-60-7 | |
| Record name | (2R,5S,13aR)-2,3,4,5,7,9,13,13a-Octahydro-8-hydroxy-7,9-dioxo-N-[(2,4,6-trifluorophenyl)methyl]-2,5-methanopyrido[1′,2′:4,5]pyrazino[2,1-b][1,3]oxazepine-10-carboxamide | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=1611493-60-7 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Bictegravir [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=1611493607 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Bictegravir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB11799 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Bictegravir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID701027937 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | BICTEGRAVIR | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/8GB79LOJ07 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Q1: What is the primary mechanism of action of bictegravir?
A1: Bictegravir is a potent integrase strand transfer inhibitor (INSTI) that acts by binding to the integrase enzyme of the Human Immunodeficiency Virus (HIV) [, , , , , ]. This binding effectively blocks the enzyme's ability to integrate viral DNA into the host cell's genome, thus preventing HIV replication [, , , , ].
Q2: How does the inhibition of HIV integrase by bictegravir affect the viral life cycle?
A2: By inhibiting HIV integrase, bictegravir disrupts a crucial step in the viral life cycle: the permanent insertion of viral DNA into the host cell's DNA [, , , ]. This prevents the production of new viral proteins and the assembly of new viral particles, effectively suppressing viral replication [, , , ].
Q3: What is the molecular formula and weight of bictegravir?
A3: The molecular formula of bictegravir is C28H27F3N4O5. Its molecular weight is 556.54 g/mol [].
Q4: What types of spectroscopic data are important for characterizing bictegravir?
A4: High-performance liquid chromatography (HPLC) coupled with mass spectrometry (MS) is commonly employed to identify and quantify bictegravir and its impurities [, , , , , ]. This technique provides precise information about the compound's mass and purity.
Q5: How does bictegravir perform under various storage conditions?
A5: Studies have evaluated the stability of bictegravir under various stress conditions to understand its degradation pathways []. This information is crucial for developing stable formulations and determining appropriate storage conditions.
Q6: What strategies have been explored to enhance the stability and bioavailability of bictegravir formulations?
A6: While specific formulation strategies for bictegravir are not detailed in the provided abstracts, researchers aim to optimize drug delivery and bioavailability [, ]. This might involve the exploration of various drug delivery systems, excipients, or formulation techniques.
Q7: What is the primary route of metabolism for bictegravir?
A7: Bictegravir is metabolized equally by cytochrome P450 3A (CYP3A) and uridine diphosphate-glucuronosyltransferase (UGT)1A1 enzymes [, , ]. This dual metabolism pathway is important to consider for potential drug-drug interactions.
Q8: How does bictegravir distribute within the body?
A8: Research indicates that bictegravir exhibits a low transfer rate across the placenta, suggesting limited fetal exposure during pregnancy []. Additionally, studies have explored its distribution in various bodily fluids, including seminal plasma, rectal fluid, and cervicovaginal fluid [].
Q9: What is known about the relationship between bictegravir plasma concentrations and its efficacy?
A9: Studies have investigated the relationship between bictegravir trough concentrations and its efficacy in suppressing HIV viral load [, , ]. Maintaining concentrations above the protein-adjusted effective concentration is crucial for sustained virologic suppression.
Q10: What types of in vitro assays are used to evaluate bictegravir's antiviral activity?
A10: Researchers commonly utilize cell-based assays to determine bictegravir's 50% effective concentration (EC50) against various HIV strains, including both HIV-1 and HIV-2 []. These assays are crucial for evaluating drug potency and assessing the potential for cross-resistance.
Q11: What animal models have been employed in bictegravir research?
A11: Mouse pregnancy models have been used to investigate the pharmacokinetics of bictegravir during pregnancy, providing insights into potential dosing strategies for pregnant women living with HIV []. Additionally, macaque models have been used to study the efficacy of bictegravir-containing regimens for pre-exposure prophylaxis (PrEP) and post-exposure prophylaxis (PEP) [].
Q12: What have clinical trials revealed about the efficacy and safety of bictegravir-containing regimens?
A12: Numerous clinical trials have demonstrated the safety and efficacy of bictegravir, typically in combination with emtricitabine and tenofovir alafenamide (B/F/TAF), for the treatment of HIV-1 infection in both treatment-naïve and treatment-experienced individuals [, , , ]. These trials have established B/F/TAF as a highly effective and well-tolerated treatment option for many people living with HIV.
Q13: What are the known mechanisms of resistance to bictegravir?
A13: While bictegravir demonstrates a high barrier to resistance, specific mutations in the HIV integrase enzyme, such as the Q148H/K/R substitutions, can reduce its susceptibility [, ]. Combinations of these mutations can lead to higher levels of resistance.
Q14: Does cross-resistance exist between bictegravir and other INSTIs?
A14: Yes, cross-resistance between bictegravir and other INSTIs, such as raltegravir, dolutegravir, and cabotegravir, can occur [, , , ]. The specific mutations that confer resistance can vary, and some mutations may have a greater impact on certain INSTIs than others.
Q15: What are the most clinically significant drug-drug interactions associated with bictegravir?
A15: Bictegravir is known to interact with drugs that are strong inhibitors or inducers of CYP3A and UGT1A1 enzymes [, ]. Notable interactions include those with metformin, a commonly used diabetes medication [], and polyvalent cations found in some nutritional supplements [].
Q16: What analytical methods are typically used to quantify bictegravir in biological samples?
A16: High-performance liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS) is a widely used technique for measuring bictegravir concentrations in biological matrices like plasma [, ]. This method offers high sensitivity and specificity, essential for accurate pharmacokinetic analysis.
Q17: What are the key parameters evaluated during the validation of analytical methods for bictegravir?
A17: Method validation ensures accuracy, precision, specificity, linearity, range, and robustness of the analytical procedure [, ]. These parameters are crucial for generating reliable and reproducible data in various experimental settings.
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。















