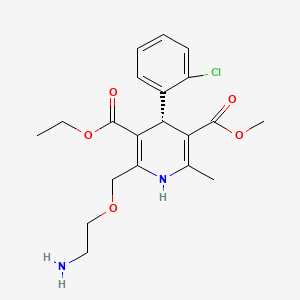
(R)-Amlodipine
概述
描述
®-Amlodipine is a chiral calcium channel blocker used primarily in the treatment of hypertension and angina. It is the enantiomer of amlodipine, which means it has a specific three-dimensional arrangement that distinguishes it from its mirror image. This compound works by inhibiting the influx of calcium ions into vascular smooth muscle and cardiac muscle, leading to vasodilation and reduced blood pressure.
准备方法
Synthetic Routes and Reaction Conditions
The synthesis of ®-Amlodipine typically involves the resolution of racemic amlodipine or asymmetric synthesis. One common method is the chiral resolution of racemic amlodipine using chiral acids or bases to separate the enantiomers. Another approach is the asymmetric synthesis, which involves the use of chiral catalysts or chiral auxiliaries to produce the desired enantiomer directly.
Industrial Production Methods
In industrial settings, the production of ®-Amlodipine often involves large-scale chiral resolution techniques. These methods are optimized for high yield and purity, ensuring that the final product meets pharmaceutical standards. The use of advanced chromatographic techniques and crystallization methods is common in the industrial production of ®-Amlodipine.
化学反应分析
Types of Reactions
®-Amlodipine undergoes several types of chemical reactions, including:
Oxidation: It can be oxidized under specific conditions to form various metabolites.
Reduction: Reduction reactions can modify the structure of ®-Amlodipine, potentially altering its pharmacological properties.
Substitution: Nucleophilic substitution reactions can occur, leading to the formation of different derivatives.
Common Reagents and Conditions
Oxidation: Common oxidizing agents include potassium permanganate and hydrogen peroxide.
Reduction: Reducing agents such as lithium aluminum hydride and sodium borohydride are used.
Substitution: Nucleophiles like amines and thiols can react with ®-Amlodipine under appropriate conditions.
Major Products Formed
The major products formed from these reactions include various metabolites and derivatives that may have different pharmacological activities. These products are often studied to understand the metabolism and potential side effects of ®-Amlodipine.
科学研究应用
Pharmacological Properties
(R)-Amlodipine is a dihydropyridine calcium channel blocker that selectively inhibits voltage-dependent L-type calcium channels. This inhibition leads to relaxation of vascular smooth muscle, resulting in decreased peripheral vascular resistance and lowered blood pressure. Its prolonged half-life allows for once-daily dosing, enhancing patient compliance .
Hypertension Management
This compound is widely recognized as a first-line treatment for hypertension. It has demonstrated significant efficacy in lowering blood pressure across various demographics, including patients with comorbid conditions such as diabetes and chronic kidney disease. Clinical trials have shown that it effectively reduces cardiovascular events compared to other antihypertensive agents .
Angina Pectoris
In patients with chronic stable angina, this compound improves exercise tolerance and reduces the frequency of anginal attacks. Its mechanism of action not only alleviates symptoms but also contributes to long-term cardiovascular health by preventing atherosclerosis progression .
Renal Protection
Studies indicate that this compound may offer protective benefits for renal function in hypertensive patients. In the ACCOMPLISH trial, it was associated with a reduced risk of progression to end-stage renal disease compared to hydrochlorothiazide .
Impact on Heart Failure
While this compound is not typically used as a primary treatment for heart failure, it has shown potential benefits in patients with preserved ejection fraction and can be safely used alongside other heart failure therapies .
Case Study 1: Amlodipine Overdose
A case study documented an adolescent girl who ingested 150 mg of amlodipine with suicidal intent. The management involved decontamination and the administration of calcium and glucagon, highlighting the challenges associated with calcium channel blocker overdoses. This case emphasizes the importance of safe storage practices for medications .
Case Study 2: COVID-19 Outcomes
A retrospective clinical investigation suggested that patients treated with amlodipine besylate had a significantly lower case fatality rate from COVID-19 compared to those not receiving the medication. This finding prompts further research into the potential protective effects of amlodipine against severe viral infections .
Comparative Efficacy
The following table summarizes key findings from various studies comparing this compound with other antihypertensive agents:
作用机制
®-Amlodipine exerts its effects by inhibiting the influx of calcium ions through L-type calcium channels in vascular smooth muscle and cardiac muscle. This inhibition leads to vasodilation, reduced peripheral resistance, and decreased blood pressure. The molecular targets of ®-Amlodipine include the alpha-1 subunit of the L-type calcium channel. By binding to this subunit, ®-Amlodipine stabilizes the channel in its inactive state, preventing calcium entry and subsequent muscle contraction.
相似化合物的比较
Similar Compounds
Amlodipine: The racemic mixture containing both ®- and (S)-enantiomers.
Nifedipine: Another calcium channel blocker with a similar mechanism of action.
Felodipine: A dihydropyridine calcium channel blocker used for similar indications.
Uniqueness
®-Amlodipine is unique due to its specific chiral configuration, which can result in different pharmacokinetic and pharmacodynamic properties compared to its (S)-enantiomer and racemic mixture. Studies have shown that ®-Amlodipine may have a more favorable side effect profile and potentially greater efficacy in certain patient populations.
Conclusion
®-Amlodipine is a valuable compound in the treatment of hypertension and angina, with distinct chemical, biological, and pharmacological properties. Its synthesis, reactions, and applications continue to be areas of active research, contributing to the development of more effective and safer medications.
生物活性
(R)-Amlodipine, a calcium channel blocker (CCB), is primarily used in the treatment of hypertension and angina. This article explores its biological activity, focusing on its pharmacological properties, mechanisms of action, clinical implications, and case studies.
Overview of Amlodipine
Amlodipine exists as two enantiomers: this compound and (S)-amlodipine. The (R)-enantiomer is generally considered more pharmacologically active. Amlodipine operates by inhibiting calcium ion influx through L-type calcium channels, leading to vasodilation and reduced vascular resistance.
- Calcium Channel Blockade : Amlodipine selectively inhibits L-type calcium channels in vascular smooth muscle and cardiac muscle.
- Vasodilation : This inhibition causes relaxation of vascular smooth muscle, leading to decreased peripheral vascular resistance and lower blood pressure.
- Cardiac Effects : While primarily a vasodilator, amlodipine also reduces myocardial oxygen demand by decreasing afterload.
Enantiomeric Differences
Research indicates that this compound exhibits different inhibitory effects on cytochrome P450 enzymes compared to its counterpart:
| Enzyme | (µM) for this compound | (µM) for (S)-Amlodipine |
|---|---|---|
| CYP3A4 | 8.22 | 14.06 |
| CYP2C9 | 12.11 | 21.45 |
| CYP2C19 | 7.12 | 10.30 |
This stereoselectivity highlights the importance of considering enantiomers in drug interactions and efficacy .
Efficacy in Hypertension
This compound has been shown to effectively lower blood pressure in hypertensive patients. In clinical trials, it demonstrated significant reductions in cardiovascular events compared to other antihypertensive therapies .
- Long-term Outcomes : Studies such as the ACCOMPLISH trial found that amlodipine significantly reduced the risk of chronic kidney disease progression and cardiovascular events compared to hydrochlorothiazide .
Case Studies
- Amlodipine Overdose : A notable case involved an 18-year-old girl who ingested 150 mg of amlodipine with suicidal intent. She exhibited severe hypotension and required multiple interventions including hyperinsulinemic-euglycemic therapy for recovery . This case emphasizes the need for careful monitoring and management of overdose situations involving CCBs.
- Vascular Health : Another study indicated that amlodipine not only lowers blood pressure but also inhibits vascular cell senescence, contributing to atherosclerosis prevention . This suggests potential benefits beyond mere blood pressure control.
In Vitro Studies
In vitro studies have demonstrated that this compound can inhibit oxidative stress-induced damage to cellular membranes, which may contribute to its protective cardiovascular effects . Additionally, it has been shown to exert anti-inflammatory effects by modulating cytokine production in various cell types.
Comparative Effectiveness
Comparative studies have shown that while both amlodipine and candesartan are effective in managing hypertension, amlodipine has a higher incidence of new-onset diabetes compared to candesartan . This highlights the importance of considering metabolic side effects when prescribing antihypertensive medications.
属性
IUPAC Name |
3-O-ethyl 5-O-methyl (4R)-2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C20H25ClN2O5/c1-4-28-20(25)18-15(11-27-10-9-22)23-12(2)16(19(24)26-3)17(18)13-7-5-6-8-14(13)21/h5-8,17,23H,4,9-11,22H2,1-3H3/t17-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
HTIQEAQVCYTUBX-QGZVFWFLSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCOC(=O)C1=C(NC(=C(C1C2=CC=CC=C2Cl)C(=O)OC)C)COCCN | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CCOC(=O)C1=C(NC(=C([C@H]1C2=CC=CC=C2Cl)C(=O)OC)C)COCCN | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C20H25ClN2O5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID90430938 | |
| Record name | (R)-Amlodipine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID90430938 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
408.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
103129-81-3 | |
| Record name | (+)-Amlodipine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=103129-81-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Amlodipine, (+)- | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0103129813 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | (R)-Amlodipine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID90430938 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | AMLODIPINE, (R)- | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/YUH55G7ZTY | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Synthesis routes and methods I
Procedure details






Synthesis routes and methods II
Procedure details





Synthesis routes and methods III
Procedure details




Synthesis routes and methods IV
Procedure details





Synthesis routes and methods V
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Q1: What is the primary mechanism of action of (R)-amlodipine?
A1: Unlike its (S)-enantiomer, this compound does not exhibit significant calcium channel blocking activity. Instead, research suggests its primary mechanism involves inhibiting smooth muscle cell migration. [, , ]
Q2: What are the downstream effects of this compound's inhibition of smooth muscle cell migration?
A2: Inhibiting smooth muscle cell migration is particularly relevant in conditions like atherosclerosis and restenosis, where the excessive proliferation and migration of these cells contribute to disease progression. [, ] By hindering this process, this compound might offer therapeutic benefits in these areas.
Q3: What is the molecular formula and weight of this compound?
A3: this compound shares the same molecular formula and weight as its racemic mixture and (S)-enantiomer. The molecular formula is C20H25ClN2O5, and the molecular weight is 408.88 g/mol.
Q4: Is there spectroscopic data available for this compound?
A4: While specific spectroscopic data for this compound might be limited due to its existence as an enantiomer within the racemic mixture, studies utilize techniques like nuclear magnetic resonance (NMR) to characterize the solvates of this compound salts. For instance, one study reports the 1H NMR spectrum of the dimethylformamide solvate of this compound L-hemitartrate. []
Q5: What are the challenges in formulating this compound for pharmaceutical use?
A5: Like many chiral drugs, formulating this compound presents challenges in achieving enantiomeric purity, stability, and optimal bioavailability. Researchers explore various strategies, including the use of specific solvents, resolving agents, and formulation techniques to overcome these hurdles. [, , , ]
Q6: How is this compound absorbed, distributed, metabolized, and excreted (ADME) in the body?
A6: While this compound lacks the calcium channel blocking activity of its (S)-enantiomer, research indicates it exhibits different pharmacokinetic properties. Studies in healthy volunteers show that this compound is more rapidly eliminated from plasma compared to (S)-amlodipine. [] Further research is needed to comprehensively understand its specific ADME profile.
Q7: Are there any in vitro studies demonstrating the effects of this compound on smooth muscle cell migration?
A7: While the provided abstracts don't offer detailed in vitro data, they highlight this compound's potent inhibition of smooth muscle cell migration. [, , ] Further research is crucial to elucidate the specific molecular mechanisms underlying this effect.
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。














