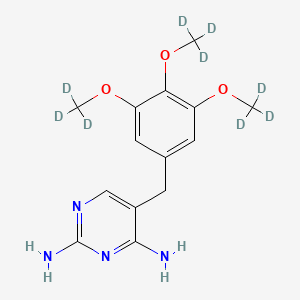
Trimethoprim-d9
描述
Trimethoprim-d9 (Major), also known as this compound (Major), is a useful research compound. Its molecular formula is C14H18N4O3 and its molecular weight is 299.378. The purity is usually 95%.
BenchChem offers high-quality this compound (Major) suitable for many research applications. Different packaging options are available to accommodate customers' requirements. Please inquire for more information about this compound (Major) including the price, delivery time, and more detailed information at [email protected].
科学研究应用
Pharmacokinetic Studies
Trimethoprim-D9 is primarily utilized as an internal standard in pharmacokinetic studies to quantify the concentration of trimethoprim and its metabolites in biological samples. The deuterated form allows for precise measurement due to its distinct mass, facilitating the differentiation from non-deuterated compounds during mass spectrometry analysis.
Table 1: Pharmacokinetic Parameters of this compound
| Parameter | Value |
|---|---|
| Cmax (µg/ml) | 2.1 ± 1.0 |
| Tmax (h) | 1.5 |
| Half-life (h) | 0.88 |
| Elimination rate (1/h) | 0.0093 ± 0.0011 |
| AUCt (µg.h/ml) | 2.901 ± 1.4 |
| Volume of distribution (l/kg) | 2.632 |
| Clearance (l/h) | 2.7 |
This data is crucial for understanding the pharmacokinetics of trimethoprim in various populations, including pediatric patients and those with renal impairment .
Clinical Applications
This compound has been employed in clinical settings to evaluate the efficacy and safety of trimethoprim-sulfamethoxazole combinations against infections such as Pneumocystis jirovecii pneumonia, particularly in immunocompromised patients.
Case Study: Treatment of Pneumocystis Jirovecii Pneumonia
A notable case involved a lymphoma patient who developed Pneumocystis jirovecii pneumonia after chemotherapy. The patient was treated with a regimen that included trimethoprim-sulfamethoxazole, leading to significant improvement in respiratory symptoms and resolution of pulmonary infiltrates as observed on follow-up imaging .
Toxicological Assessments
The use of this compound extends to toxicological studies where it serves as a reference standard for assessing the safety profiles of trimethoprim and its metabolites. Research indicates that certain metabolites can form reactive protein adducts, which are crucial for understanding adverse drug reactions .
Table 2: Toxicological Findings Related to Trimethoprim Metabolites
| Metabolite | Toxicity Observed |
|---|---|
| Sulfamethoxazole-N-acetyl | Potential nephrotoxicity |
| Trimethoprim | Rare cases of hematologic toxicity |
These findings underscore the importance of monitoring drug levels in patients to mitigate risks associated with elevated concentrations of these compounds .
Method Development for Analysis
Recent advancements have led to the development of robust analytical methods using liquid chromatography-tandem mass spectrometry (LC-MS/MS) for the simultaneous quantification of trimethoprim and sulfamethoxazole in serum and plasma samples.
Table 3: Analytical Method Validation Parameters
| Parameter | Result |
|---|---|
| Linearity | R² > 0.99 |
| Precision (CV %) | < 5% |
| Recovery (%) | 90-110% |
| Stability | Up to 120 hours |
This method has been validated according to FDA guidelines, ensuring its applicability for routine therapeutic drug monitoring and clinical studies focused on optimizing treatment regimens for various infectious diseases .
作用机制
Target of Action
Trimethoprim-d9, also known as this compound (Major), primarily targets the bacterial enzyme dihydrofolate reductase (DHFR) . DHFR is a critical enzyme that catalyzes the formation of tetrahydrofolic acid (THF), an essential precursor in the biosynthesis of nucleic acids .
Mode of Action
this compound inhibits DHFR, thereby preventing the synthesis of bacterial DNA and ultimately leading to bacterial death . It binds with a much stronger affinity to bacterial DHFR compared to its mammalian counterpart, allowing this compound to selectively interfere with bacterial biosynthetic processes .
Biochemical Pathways
The inhibition of DHFR by this compound disrupts the biosynthesis pathways of thymidylate and purines, as well as several other amino acids like glycine, methionine, serine, and N-formyl-methionyl tRNA . This leads to an imbalance in the pathways involved in active synthesizing thymidylate, disrupts DNA replication, and eventually causes cell death .
Pharmacokinetics
this compound is a potent inhibitor of multidrug and toxin extrusion protein (MATE) and a weak inhibitor of cytochrome P450 (CYP) 2C8 . These properties can influence the absorption, distribution, metabolism, and excretion (ADME) of the compound, impacting its bioavailability .
Result of Action
The molecular and cellular effects of this compound’s action include the inhibition of bacterial DNA synthesis, leading to bacterial death . Some of the new analogs of this compound inhibited DHFR activity more strongly than Trimethoprim did, indicating that the addition of amide bonds into the analogs of this compound increases their affinity towards DHFR .
Action Environment
Environmental factors can influence the action, efficacy, and stability of this compound. For instance, pH plays a role in the mode of action of this compound on Escherichia coli . Moreover, soil-related factors, animal husbandry and waste management, potable and wastewater, and food safety can contribute to antimicrobial resistance . These factors need to be considered in modeling the fate and transport of this compound in coastal/estuarine waters .
生化分析
Biochemical Properties
Trimethoprim-d9 interacts with the enzyme dihydrofolate reductase (DHFR), which plays a crucial role in the biosynthesis pathways of thymidylate, purines, and several amino acids . The interaction between this compound and DHFR inhibits the enzyme’s activity, disrupting DNA replication and eventually leading to cell death .
Cellular Effects
The effects of this compound on cells are primarily due to its inhibition of DHFR. This disruption in folate metabolism leads to an imbalance in the pathways involved in synthesizing thymidylate, which is essential for DNA replication . As a result, the function of cells is significantly affected, leading to cell death .
Molecular Mechanism
This compound exerts its effects at the molecular level by binding to the DHFR enzyme. This binding inhibits the enzyme’s activity, preventing the reduction of dihydrofolate acid to tetrahydrofolic acid . This disruption in folate metabolism leads to an imbalance in the pathways involved in synthesizing thymidylate, which is essential for DNA replication .
Metabolic Pathways
This compound is involved in the folate metabolism pathway. It interacts with the DHFR enzyme, which catalyzes the reduction of dihydrofolate acid to tetrahydrofolic acid . The inhibition of DHFR by this compound disrupts this metabolic pathway, leading to an imbalance in the pathways involved in synthesizing thymidylate .
生物活性
Trimethoprim-d9 (Major) is a deuterated analog of trimethoprim, a well-known antibiotic primarily used for treating bacterial infections. The deuteration enhances its tracking in biological studies, providing insights into its pharmacokinetics and metabolic pathways. This article explores the biological activity of this compound, focusing on its mechanism of action, efficacy against various pathogens, and relevant research findings.
This compound functions as a reversible inhibitor of dihydrofolate reductase (DHFR), an enzyme essential for synthesizing tetrahydrofolic acid (THF) from dihydrofolate (DHF). This inhibition disrupts the production of nucleic acids and proteins in bacteria, leading to their growth inhibition and eventual cell death.
- Selectivity : this compound exhibits a higher affinity for bacterial DHFR compared to mammalian DHFR, which allows it to selectively target bacterial biosynthetic processes without significantly affecting human cells .
Efficacy Against Bacterial Strains
This compound has shown effectiveness against a range of bacterial pathogens. Its performance can be evaluated through various assays:
| Pathogen | Efficacy (IC50) | Reference |
|---|---|---|
| Escherichia coli | 55.26 µM | |
| Staphylococcus aureus | 10.5 µM | |
| Klebsiella pneumoniae | 30 µM |
The IC50 values indicate the concentration required to inhibit 50% of the bacterial growth, showcasing this compound's potential as an effective antibacterial agent.
Pharmacokinetics
The pharmacokinetic profile of this compound is similar to that of its parent compound:
- Absorption : Achieves peak serum concentrations within 1-4 hours post-administration.
- Distribution : Extensively distributed in body tissues, including sputum and vaginal fluids.
- Metabolism : Primarily metabolized by CYP enzymes, with about 10-20% excreted as metabolites .
Case Studies and Research Findings
Several studies have explored the biological activity and potential applications of this compound:
- Inhibition Studies : A study demonstrated that this compound effectively inhibits DHFR in various bacterial strains, with some analogs showing improved potency compared to traditional trimethoprim .
- Resistance Mechanisms : Research indicates that this compound can be utilized to study bacterial resistance mechanisms due to its selective inhibition profile. This is crucial for developing new strategies to combat antibiotic resistance .
- Combination Therapy : this compound is often studied in combination with sulfamethoxazole, which inhibits an earlier step in folate synthesis, leading to synergistic effects against resistant bacterial strains .
属性
IUPAC Name |
5-[[3,4,5-tris(trideuteriomethoxy)phenyl]methyl]pyrimidine-2,4-diamine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18)/i1D3,2D3,3D3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
IEDVJHCEMCRBQM-GQALSZNTSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
COC1=CC(=CC(=C1OC)OC)CC2=CN=C(N=C2N)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
[2H]C([2H])([2H])OC1=CC(=CC(=C1OC([2H])([2H])[2H])OC([2H])([2H])[2H])CC2=CN=C(N=C2N)N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C14H18N4O3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID10662219 | |
| Record name | Trimethoprim-d9 | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID10662219 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
299.37 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
1189460-62-5 | |
| Record name | Trimethoprim-d9 | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID10662219 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Synthesis routes and methods I
Procedure details








Synthesis routes and methods II
Procedure details





体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。













