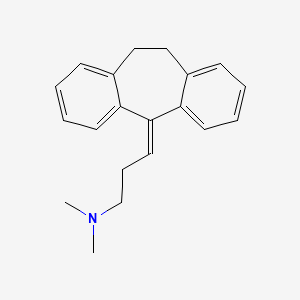
阿米替林
概述
描述
作用机制
科学研究应用
Depression Treatment
Amitriptyline is primarily recognized for its efficacy in treating major depressive disorder. A meta-analysis of randomized controlled trials indicated that amitriptyline has a slightly higher response rate compared to other antidepressants, including selective serotonin reuptake inhibitors (SSRIs) and other tricyclic antidepressants . The odds ratio favored amitriptyline, suggesting its effectiveness in alleviating depressive symptoms.
Neuropathic Pain Management
Amitriptyline is frequently prescribed off-label for neuropathic pain conditions, such as diabetic neuropathy and postherpetic neuralgia. Research has shown that amitriptyline can reduce pain intensity and improve quality of life in patients suffering from these chronic pain syndromes. A systematic review highlighted its role as a first-line treatment option for neuropathic pain .
Case Study: Diabetic Neuropathy
In a clinical study involving diabetic patients, those treated with amitriptyline reported significant reductions in pain scores compared to placebo groups. The drug's mechanism appears to involve modulation of neurotransmitter levels and inhibition of pain pathways in the central nervous system .
Fibromyalgia
Fibromyalgia is another condition where amitriptyline has shown promise. Studies indicate that low-dose amitriptyline can help alleviate fibromyalgia symptoms, including widespread pain and sleep disturbances. A randomized controlled trial demonstrated that patients receiving amitriptyline experienced significant improvements in their fibromyalgia impact scores compared to those receiving placebo .
Migraine Prophylaxis
Amitriptyline is also utilized for migraine prevention. Clinical evidence supports its efficacy in reducing the frequency and severity of migraine attacks. A study found that patients taking amitriptyline had fewer migraine days per month compared to those on placebo, making it a valuable option for chronic migraine sufferers .
Irritable Bowel Syndrome (IBS)
Recent research has explored the use of amitriptyline in managing irritable bowel syndrome symptoms. A large trial indicated that low-dose amitriptyline significantly improved IBS symptom scores after six months of treatment, demonstrating its potential as an effective therapy for this condition .
Anxiety Disorders
Amitriptyline's anxiolytic properties have led to its use in treating anxiety disorders, particularly when these conditions co-occur with depression or chronic pain syndromes. While not first-line therapy for anxiety alone, it can be beneficial in complex cases where multiple symptoms overlap .
Chronic Pain Syndromes
Beyond neuropathic pain, amitriptyline has been investigated for various chronic pain conditions, including complex regional pain syndrome (CRPS) and tension-type headaches. Its ability to modulate pain perception through central mechanisms makes it a relevant option in these contexts .
Summary Table of Amitriptyline Applications
| Condition | Evidence Level | Key Findings |
|---|---|---|
| Depression | High | Effective as a first-line treatment |
| Neuropathic Pain | High | Reduces pain intensity; enhances quality of life |
| Fibromyalgia | Moderate | Improves symptom scores; enhances sleep quality |
| Migraine Prophylaxis | Moderate | Decreases frequency and severity of attacks |
| Irritable Bowel Syndrome | High | Significant symptom improvement over placebo |
| Anxiety Disorders | Moderate | Beneficial in co-morbid cases |
| Chronic Pain Syndromes | Moderate | Effective in various chronic pain conditions |
生化分析
Biochemical Properties
Amitriptyline works by increasing the synaptic concentration of serotonin and/or norepinephrine in the central nervous system by inhibiting their reuptake by the presynaptic neuronal membrane pump . This action prolongs the sympathetic activity of these biogenic amines .
Cellular Effects
Amitriptyline has been shown to interfere with the formation of aggresome-like aggregates and autophagy-mediated clearance of aggregates . It also has immunomodulatory effects, such as lowering the production of typical T H 2-cytokines in T lymphocytes of asthmatic mice .
Molecular Mechanism
Amitriptyline’s key mechanism of action lies in the elevation of extracellular biogenic amine levels, notably those of noradrenaline and serotonin, by its blockade of cellular noradrenaline and serotonin reuptake transporters . This response may underlie chronic amitriptyline action on dopamine and norepinephrine neurotransmission .
Temporal Effects in Laboratory Settings
You may have flu-like symptoms like feeling sick, muscle pain, and feeling tired or restless . To help prevent this from happening, your doctor will probably recommend reducing your dose gradually over several weeks .
Dosage Effects in Animal Models
In animal models, oral administration of amitriptyline provides a valid model for behavioral assessment of antidepressant-like effects . The effectiveness of oral amitriptyline varies depending on sex, duration of treatment, and the depression model used .
Metabolic Pathways
Amitriptyline is readily absorbed in the GI tract and subject to extensive hepatic metabolism with less than 5% of the drug eliminated unchanged . The main metabolizing enzymes with clinical significance for amitriptyline are CYP2C19 and CYP2D6 .
Transport and Distribution
Amitriptyline is transported and distributed within cells and tissues via absorption in the GI tract . It is subject to extensive hepatic metabolism .
Subcellular Localization
Given its mechanism of action, it is likely that Amitriptyline localizes to the synapses in neurons where it can inhibit the reuptake of serotonin and norepinephrine .
准备方法
合成路线和反应条件: 阿米替林可以通过多种方法合成。一种常见的方法是二苯并环庚酮与二甲胺在还原剂(如氢化锂铝)存在下反应。 该反应通过形成中间体进行,然后环化生成阿米替林 .
工业生产方法: 在工业环境中,阿米替林通常通过多步合成工艺生产,以确保高产量和纯度。 该过程涉及使用高性能液相色谱 (HPLC) 等先进技术进行纯化和质量控制 .
化学反应分析
反应类型: 阿米替林会发生多种化学反应,包括:
氧化: 阿米替林可以氧化生成其 N-氧化衍生物。
还原: 阿米替林的还原会导致仲胺的形成。
常见试剂和条件:
氧化: 常见的氧化剂包括过氧化氢和过酸。
还原: 常用的还原剂包括氢化锂铝或硼氢化钠。
主要形成的产物:
氧化: 形成 N-氧化衍生物。
还原: 形成仲胺。
取代: 形成取代的阿米替林衍生物.
相似化合物的比较
阿米替林经常与其他三环抗抑郁药进行比较,例如:
诺特里普替林: 结构相似,但副作用更少。
地昔帕明: 以对去甲肾上腺素再摄取抑制的选择性更高而闻名。
丙咪嗪: 用于类似适应症,但其副作用特征不同。
生物活性
Amitriptyline is a tricyclic antidepressant (TCA) that has been widely used for the treatment of major depressive disorder and various pain conditions. Its biological activity extends beyond its antidepressant effects, involving complex interactions at the cellular and molecular levels. This article delves into the various aspects of amitriptyline's biological activity, including its mechanisms of action, effects on cell viability, autophagy modulation, and additional pharmacological properties.
Amitriptyline primarily functions by inhibiting the reuptake of neurotransmitters, specifically serotonin and norepinephrine, thereby enhancing their availability in the synaptic cleft. This mechanism is crucial for its antidepressant effects and is mediated through the following pathways:
- Serotonin Transporter (SERT) Inhibition : Amitriptyline blocks SERT, leading to increased serotonin levels.
- Norepinephrine Transporter (NET) Inhibition : It also inhibits NET, enhancing norepinephrine availability.
- Receptor Binding : Amitriptyline exhibits strong binding affinities for various receptors, including:
- Alpha-adrenergic receptors
- Histamine (H1) receptors
- Muscarinic (M1) receptors
These interactions contribute to its sedative effects and anticholinergic properties, which are more pronounced compared to other TCAs .
Effects on Cell Viability and Proliferation
Recent studies have shown that amitriptyline affects cell viability in neuroblastoma cell lines (SH-SY5Y). Notably, it induces a concentration- and time-dependent reduction in cell viability. Key findings include:
- Cell Viability Reduction : At concentrations of 50 μM, cell viability decreased significantly over time; specifically, 81.03% at 24 hours, dropping to 43.60% by 72 hours .
- Clonogenic Capacity : Amitriptyline treatment reduced the clonogenic capacity of SH-SY5Y cells, indicating its potential cytotoxic effects .
Autophagy Modulation
Amitriptyline has been found to modulate autophagy in treated cells. However, its cytotoxic effects appear to be independent of autophagy modulation:
- Autophagy Inhibition Studies : When SH-SY5Y cultures were pre-treated with chloroquine (an autophagy inhibitor), amitriptyline's effects on cell viability remained consistent, suggesting that its cytotoxicity does not rely on altering autophagic processes .
- Lysosomal Accumulation : Amitriptyline induced lysosomal accumulation without affecting lysosomal pH, further supporting its complex interaction with cellular homeostasis .
Additional Pharmacological Properties
Beyond its antidepressant activity, amitriptyline exhibits several other biological activities:
- Antimicrobial Activity : Studies indicate that amitriptyline possesses significant antibacterial properties against both Gram-positive and Gram-negative bacteria. In vivo experiments demonstrated a reduction in bacterial counts in mice treated with amitriptyline after exposure to Salmonella typhimurium, highlighting its potential as an antimicrobial agent .
| Study Type | Findings |
|---|---|
| In Vivo Study | Significant reduction in bacterial counts in treated mice (p<0.01) |
| In Vitro Study | Bacteriostatic effects against various bacterial strains |
Case Studies and Clinical Implications
Amitriptyline's off-label use has been documented extensively. For instance:
- Chronic Pain Management : Amitriptyline is frequently prescribed for neuropathic pain management due to its analgesic properties.
- Sleep Disorders : Its sedative effects make it a common choice for treating insomnia associated with depression.
属性
IUPAC Name |
N,N-dimethyl-3-(2-tricyclo[9.4.0.03,8]pentadeca-1(15),3,5,7,11,13-hexaenylidene)propan-1-amine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C20H23N/c1-21(2)15-7-12-20-18-10-5-3-8-16(18)13-14-17-9-4-6-11-19(17)20/h3-6,8-12H,7,13-15H2,1-2H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
KRMDCWKBEZIMAB-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN(C)CCC=C1C2=CC=CC=C2CCC3=CC=CC=C31 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C20H23N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Related CAS |
17086-03-2 (pamoate (2:1)), 30227-34-0 (maleate (1:1)), 549-18-8 (hydrochloride) | |
| Record name | Amitriptyline [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000050486 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
DSSTOX Substance ID |
DTXSID7022594 | |
| Record name | Amitriptyline | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7022594 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
277.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Amitriptyline | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014466 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Boiling Point |
410.26°C (rough estimate) | |
| Record name | Amitriptyline | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00321 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Solubility |
freely soluble in water, In water, 9.71 mg/L at 24 °C, 4.50e-03 g/L | |
| Record name | Amitriptyline | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00321 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | AMITRIPTYLINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3007 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Amitriptyline | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014466 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
The mechanism of action of this drug is not fully elucidated. It is suggested that amitriptyline inhibits the membrane pump mechanism responsible for the re-uptake of transmitter amines, such as norepinephrine and serotonin, thereby increasing their concentration at the synaptic clefts of the brain,. These amines are important in regulating mood. The monoamine hypothesis in depression, one of the oldest hypotheses, postulates that deficiencies of serotonin (5-HT) and/or norepinephrine (NE) neurotransmission in the brain lead to depressive effects. This drug counteracts these mechanisms, and this may be the mechanism of amitriptyline in improving depressive symptoms. Whether its analgesic effects are related to its mood-altering activities or attributable to a different, less obvious pharmacological action (or a combination of both) is unknown., Acute and chronic effects of the antidepressant drugs tranylcypromine, a monoamine oxidase inhibitor, and amitriptyline, a monoamine uptake inhibitor, were studied on beta-adrenergic receptor function in mouse astrocytes in primary cultures. In clinically relevant concentrations, acute administration of either antidepressant drug had a direct inhibitory effect on the binding of the beta-adrenergic ligand dihydroalprenolol and on the isoproterenol-induced accumulation of cyclic AMP. However, in the absence of isoproterenol, these drugs enhanced the formation of cyclic AMP in the astrocytes. Chronic exposure to amitriptyline or tranylcypromine led to a decrease in isoproterenol-induced accumulation of cyclic AMP, and the time course for the development of this phenomenon was similar to that reported for whole brain in vivo. These findings suggest that these antidepressant drugs act as a partial agonists at beta-adrenergic receptors on astrocytes, and that the down-regulation of beta-adrenergic activity that occurs in vivo after chronic administration of antidepressant drugs may, to a large extent, take place in astrocytes and may result from the partial beta-agonist nature of the drugs., Astrocytes play important roles in guiding the construction of the nervous system, controlling extracellular ions and neurotransmitters, and regulating CNS synaptogenesis. Egr-1 is a transcription factor involved in neuronal differentiation and astrocyte cell proliferation. In this study, we investigated whether the tricyclic antidepressant (TCA) amitriptyline induces Egr-1 expression in astrocytes using rat C6 glioma cells as a model. We found that amitriptyline increased the expression of Egr-1 in a dose- and time-dependent manner. The amitriptyline-induced Egr-1 expression was mediated through serum response elements (SREs) in the Egr-1 promoter. SREs were activated by the Ets-domain transcription factor Elk-1 through the ERK and JNK mitogen-activated protein (MAP) kinase pathways. The inhibition of the ERK and JNK MAP kinase signals attenuated amitriptyline-induced transactivation of Gal4-Elk-1 and Egr-1 promoter activity. Our findings suggest that the induction of Egr-1 expression in astrocytes may be required to attain the therapeutic effects of antidepressant drugs., Antidepressants such as serotonin-noradrenaline reuptake inhibitors (SNRIs) and tricyclic antidepressants (TCAs) are frequently used for the management of neuropathic pain. Noradrenaline (NA) and serotonin (5-HT) increase in the spinal cord by reuptake inhibition is considered to be main mechanism of the therapeutic effect of antidepressants in neuropathic pain. In the present study, we examined the analgesic effects of duloxetine (SNRI) and amitriptyline (TCA) in a rat model of neuropathic pain induced by spinal nerve ligation (SNL). Intraperitoneal administration of duloxetine and amitriptyline dose-dependently (3,10 and 30 mg/kg) suppressed hyperalgesia induced by SNL. In vivo microdialysis in the lumbar spinal dorsal horn revealed that NA and 5-HT concentrations increased after intraperitoneal administration of duloxetine and amitriptyline (10 mg/kg, respectively). We further determined NA and 5-HT contents in homogenized samples from the ipsilateral dorsal spinal cord after SNL. Although the NA content in SNL rats 2 weeks after ligation was higher than that in SNL rats 4 weeks after ligation, the analgesic efficacy of duloxetine and amitriptyline was similar between two groups. The present study suggests that NA/5-HT increase in the spinal cord is crucial in the antihyperalgesic effect of duloxetine and amitriptyline. The plastic change of the descending noradrenergic system does not obviously affect the analgesic efficacy of duloxetine and amitriptyline., Recent studies show that neuronal and glial plasticity are important for the therapeutic action of antidepressants. Here, we demonstrated that amitriptyline, a tricyclic antidepressant, significantly increased GDNF mRNA and GDNF release in C6 cells. Furthermore, different classes of antidepressants increased GDNF release, but non-antidepressant psychotropic drugs did not. The amitriptyline-induced GDNF release was completely inhibited by U0126, a mitogen-activated protein kinase (MAPK)-extracellular signal-regulated kinase (ERK) kinase (MEK) inhibitor, but was not inhibited by H-89, a protein kinase A inhibitor or calphostin C, a protein kinase C inhibitor. These results suggest that the amitriptyline-induced GDNF release may be regulated through a MEK/MAPK pathway. Next, we examined the effects of monoamines on GDNF release, because antidepressants are known to increase monoamines. 5-HT increased GDNF mRNA and GDNF release, but noradrenaline and dopamine did not. The 5-HT-induced GDNF release was partially, but significantly, blocked by ketanserin, a 5-HT2A receptor antagonist. The 5-HT-induced GDNF release was completely inhibited by U0126, but was not inhibited by H-89 or calphostin C. These results suggest that the 5-HT-induced GDNF release was mediated through a MEK/MAPK pathway and, at least, 5-HT2A receptors. GDNF, as well as other neurotrophic factors, may contribute to explain the therapeutic action of antidepressants and suggest a novel strategy of pharmacological intervention., For more Mechanism of Action (Complete) data for AMITRIPTYLINE (13 total), please visit the HSDB record page. | |
| Record name | Amitriptyline | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00321 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | AMITRIPTYLINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3007 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals | |
CAS No. |
50-48-6 | |
| Record name | Amitriptyline | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=50-48-6 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Amitriptyline [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000050486 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Amitriptyline | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00321 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Amitriptyline | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7022594 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Amitriptyline | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.038 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | AMITRIPTYLINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/1806D8D52K | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | AMITRIPTYLINE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3007 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Amitriptyline | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014466 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
196-197, 196 - 197 °C | |
| Record name | Amitriptyline | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00321 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Amitriptyline | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014466 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details








Synthesis routes and methods II
Procedure details






Synthesis routes and methods III
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。














