艾尔维格雷韦
描述
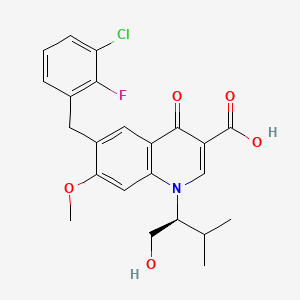
Elvitegravir is a quinolinemonocarboxylic acid that is 7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid substited at position 1 by a 1-hydroxy-3-methylbutan-2-yl group and at position 6 by a 3-chloro-2-fluorobenzyl group (the S-enantiomer). It is used in combination therapy for the treatment of HIV-1 infection. It has a role as a HIV-1 integrase inhibitor. It is a quinolinemonocarboxylic acid, an organofluorine compound, an aromatic ether, a quinolone and a member of monochlorobenzenes.
Elvitegravir is a human immunodeficiency virus type 1 (HIV-1) integrase strand transfer inhibitor (INSTI) used for the treatment of HIV-1 infection in antiretroviral treatment-experienced adults. Because integrase is necessary for viral replication, inhibition prevents the integration of HIV-1 DNA into the host genome and thereby blocks the formation of the HIV-1 provirus and resulting propagation of the viral infection. Although available as a single dose tablet, elvitegravir must be used in combination with an HIV protease inhibitor coadministered with ritonavir and another antiretroviral drug. Elvitegravir was first licensed from Japan Tobacco in 2008 and developed by Gilead Sciences. It was FDA approved on August 27, 2012. On September 24, 2014, the FDA approved the single pill form of elvitegravir.
Elvitegravir is a Human Immunodeficiency Virus Integrase Strand Transfer Inhibitor. The mechanism of action of elvitegravir is as a HIV Integrase Inhibitor, and Cytochrome P450 2C9 Inducer.
Elvitegravir is a modified quinolone antibiotic with activity against human immunodeficiency virus 1. Elvitegravir is an inhibitor of viral integrase and retains activity against integrase mutants that are resistant to Raltegravir.
See also: ... View More ...
科学研究应用
Clinical Applications
1. Treatment of HIV-1 Infection
Elvitegravir is commonly used in combination with other antiretroviral agents to treat HIV-1 infection. In pivotal Phase 3 studies, elvitegravir combined with cobicistat, emtricitabine, and tenofovir disoproxil fumarate was shown to be non-inferior to other regimens like ritonavir-boosted atazanavir. The combination therapy demonstrated sustained viral suppression and improved patient tolerability compared to traditional therapies .
2. Efficacy in Treatment-Naïve Patients
In treatment-naïve adults, elvitegravir has been incorporated into once-daily single-tablet regimens such as Genvoya and Stribild. These formulations have shown significant efficacy, with studies indicating that over 90% of patients achieved undetectable viral loads after 96 weeks of treatment . The safety profile of elvitegravir is favorable, with lower incidences of renal and bone-related side effects compared to older regimens .
Innovative Drug Delivery Systems
1. Nanoformulations for Enhanced Delivery
Recent research has focused on developing innovative nanoformulations of elvitegravir to improve its delivery across biological barriers, particularly the blood-brain barrier (BBB). A study demonstrated that a poloxamer-PLGA nanoparticle formulation significantly enhanced the intracellular uptake of elvitegravir in HIV-infected macrophages and improved its ability to penetrate the BBB without compromising its integrity . This advancement holds promise for treating HIV-associated neurocognitive disorders by ensuring effective drug levels in the central nervous system.
2. Combination with Curcumin
Another study explored the use of curcumin as an adjuvant to enhance the delivery of elvitegravir across the BBB. Findings indicated that curcumin increased the concentration of elvitegravir in brain tissues when administered via intranasal routes, suggesting a potential strategy for improving therapeutic outcomes in HIV neuropathogenesis . This approach could lead to more effective treatments for patients experiencing cognitive impairments related to HIV.
Case Studies and Clinical Trials
1. Phase 3 Trials
Elvitegravir has been evaluated in numerous clinical trials assessing its efficacy and safety. For instance, a randomized Phase 3 trial comparing elvitegravir with raltegravir showed comparable virologic suppression rates after 48 weeks, highlighting its effectiveness as a treatment option for experienced patients .
2. Long-term Efficacy Studies
Longitudinal studies have demonstrated that patients on elvitegravir-based regimens maintain viral suppression over extended periods, with data showing sustained efficacy beyond two years . These studies emphasize the importance of adherence to therapy and monitoring for potential drug resistance.
Summary Table: Key Findings on Elvitegravir
| Application Area | Key Findings |
|---|---|
| HIV Treatment | Effective in treatment-naïve and experienced patients; high rates of viral suppression achieved. |
| Nanoformulation | Improved BBB penetration and intracellular uptake in HIV-infected cells; potential for HAND treatment. |
| Adjuvant Therapy | Curcumin enhances brain delivery of elvitegravir; may improve outcomes in neurological conditions. |
| Clinical Trial Evidence | Demonstrated long-term efficacy and safety; comparable outcomes to other antiretroviral therapies. |
作用机制
Target of Action
Elvitegravir is an antiretroviral agent that primarily targets the HIV-1 integrase . This enzyme is encoded by the HIV-1 virus and is essential for viral replication .
Mode of Action
Elvitegravir acts as an integrase strand transfer inhibitor (INSTI) . The integrase enzyme is responsible for integrating the HIV-1 DNA into the host genome. By inhibiting this enzyme, elvitegravir prevents the integration of HIV-1 DNA into the host genome, thereby blocking the formation of the HIV-1 provirus and the propagation of the viral infection .
Biochemical Pathways
The primary biochemical pathway affected by elvitegravir is the HIV-1 replication cycle . By inhibiting the integrase enzyme, elvitegravir disrupts the integration of the viral DNA into the host genome, a crucial step in the HIV-1 replication cycle . This results in the prevention of the formation of new HIV-1 proviruses, thereby halting the propagation of the viral infection .
Pharmacokinetics
Elvitegravir undergoes primarily oxidative metabolism via CYP3A , and is secondarily glucuronidated via UGT1A1/3 enzymes . The metabolites of elvitegravir are found in the plasma at very low concentrations and display considerably lower anti-HIV activity . Elvitegravir’s metabolism primarily occurs via cytochrome P450 3A4 (CYP3A4) and requires pharmacokinetic boosting to achieve systemic exposures amenable to once-daily dosing .
Result of Action
The molecular effect of elvitegravir’s action is the inhibition of the HIV-1 integrase enzyme, which prevents the integration of HIV-1 DNA into the host genome . On a cellular level, this results in the blocking of the formation of the HIV-1 provirus and the propagation of the viral infection . This effectively halts the replication of the HIV-1 virus within the host cells .
Action Environment
Environmental factors such as the presence of other drugs can influence the action of elvitegravir. For instance, elvitegravir must be used in combination with an HIV protease inhibitor coadministered with ritonavir and another antiretroviral drug . Additionally, substances that induce CYP3A can reduce elvitegravir concentrations in the body, potentially triggering the development of resistant virus strains . Furthermore, decreased levels of plasma albumin, which occur during pregnancy, can enhance the hepatic clearance of elvitegravir, as it binds strongly to plasma albumin .
生物活性
Elvitegravir (EVG) is an integrase strand transfer inhibitor (INSTI) used in the treatment of HIV-1 infection. It is often co-formulated with other antiretroviral agents, such as cobicistat, emtricitabine, and tenofovir alafenamide, in single-tablet regimens. This article delves into the biological activity of elvitegravir, highlighting its efficacy, safety, pharmacokinetics, and resistance profiles based on diverse clinical studies.
Elvitegravir functions by inhibiting the integrase enzyme, which is crucial for the integration of viral DNA into the host genome. By blocking this process, elvitegravir effectively prevents the replication of HIV-1. Its potency is reflected in its ability to suppress viral loads significantly in both treatment-naive and treatment-experienced populations.
Efficacy
Clinical trials have demonstrated elvitegravir's efficacy in achieving virologic suppression. A notable study involving a single-tablet regimen (E/C/F/TAF) showed that 93% of Asian participants achieved sustained viral suppression over 96 weeks . In a separate trial focusing on HIV-2-infected individuals in Senegal, 93.3% of subjects had viral loads suppressed to below 50 copies/mL after 48 weeks of treatment .
Table 1: Summary of Efficacy Data from Key Studies
Safety Profile
Elvitegravir is generally well-tolerated with a favorable safety profile. In clinical trials, it has shown minimal side effects and no specific laboratory monitoring requirements . The most common adverse events reported include gastrointestinal disturbances and headache, which are typically mild to moderate in severity.
Case Study: Long-term Safety Assessment
In a long-term study assessing the safety and efficacy of elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate (E/C/F/TDF), participants were monitored for up to 144 weeks. The results indicated infrequent development of drug resistance among treatment-naive patients, highlighting elvitegravir's robustness as a therapeutic option .
Pharmacokinetics
Elvitegravir undergoes metabolic processing primarily through the cytochrome P450 3A4 pathway and is also subject to glucuronidation via UGT1A1 and UGT1A3 enzymes. Studies show that it achieves higher trough concentrations when boosted with ritonavir or cobicistat compared to unboosted dosing .
Table 2: Pharmacokinetic Properties of Elvitegravir
| Parameter | Value |
|---|---|
| Plasma Protein Binding | 98-99% |
| Metabolism | CYP3A4/5 (major), UGT1A1/3 (minor) |
| Excretion | 94.8% fecal, 6.7% urinary |
Resistance Profile
The emergence of drug resistance remains a critical concern in HIV therapy. Elvitegravir has shown a low incidence of resistance mutations among treatment-naive patients . However, specific mutations (e.g., G140S and Q148R) can confer resistance in some cases, particularly in patients with pre-existing drug-resistant strains .
属性
IUPAC Name |
6-[(3-chloro-2-fluorophenyl)methyl]-1-[(2S)-1-hydroxy-3-methylbutan-2-yl]-7-methoxy-4-oxoquinoline-3-carboxylic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C23H23ClFNO5/c1-12(2)19(11-27)26-10-16(23(29)30)22(28)15-8-14(20(31-3)9-18(15)26)7-13-5-4-6-17(24)21(13)25/h4-6,8-10,12,19,27H,7,11H2,1-3H3,(H,29,30)/t19-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
JUZYLCPPVHEVSV-LJQANCHMSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)C(CO)N1C=C(C(=O)C2=C1C=C(C(=C2)CC3=C(C(=CC=C3)Cl)F)OC)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC(C)[C@@H](CO)N1C=C(C(=O)C2=C1C=C(C(=C2)CC3=C(C(=CC=C3)Cl)F)OC)C(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C23H23ClFNO5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID101021650 | |
| Record name | Elvitegravir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID101021650 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
447.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Solubility |
<0.3 mcg/mL | |
| Record name | Elvitegravir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09101 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
Mechanism of Action |
Elvitegravir is an HIV-1 integrase strand transfer inhibitor (INSTI). Integrase is an HIV-1 encoded enzyme that is required for viral replication. Inhibition of integrase prevents the integration of HIV-1 DNA into host genomic DNA, blocking the formation of the HIV-1 provirus and propagation of the viral infection. Elvitegravir does not inhibit human topoisomerases I or II. | |
| Record name | Elvitegravir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09101 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
697761-98-1 | |
| Record name | Elvitegravir | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=697761-98-1 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Elvitegravir [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0697761981 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Elvitegravir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09101 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Elvitegravir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID101021650 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 6-(3-chloro-2-fluorobenzyl)-1-[(2S)-1-hydroxy-3-methylbutan-2-yl]-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | ELVITEGRAVIR | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/4GDQ854U53 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。















