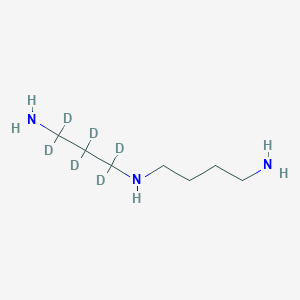
Spermidine-d6
Overview
Description
- It plays essential roles in cell growth, gene expression, and cellular processes.
- This compound serves as an internal standard for quantifying natural spermidine using GC- or LC-MS methods .
Spermidine-d6: (CAS 2514812-10-1) is a deuterium-labeled form of , an endogenous polyamine.
Preparation Methods
Synthetic Routes: Spermidine-d6 is synthesized by incorporating deuterium-labeled precursors during its formation.
Reaction Conditions: The specific synthetic route and conditions are not provided in the available information.
Industrial Production: Details regarding industrial-scale production methods are scarce.
Chemical Reactions Analysis
Reactions: Spermidine-d6 can undergo various chemical reactions, including oxidation, reduction, and substitution.
Common Reagents: Specific reagents are not mentioned, but typical polyamine reaction conditions apply.
Major Products: The primary products depend on the specific reaction and starting materials.
Scientific Research Applications
Role in Aging and Longevity
Spermidine has been associated with promoting longevity and mitigating age-related diseases. Research indicates that spermidine supplementation can enhance autophagy, a cellular process essential for degrading and recycling cellular components. This process is crucial in combating aging-related cellular damage.
Key Findings:
- Spermidine induces the expression of autophagy-related genes (e.g., Atg7, Atg15) and transcription factors like TFEB, which are vital for autophagic processes .
- Studies have shown that spermidine can prolong lifespan in model organisms by enhancing cellular resilience against stressors .
Hair Growth Stimulation
Spermidine has demonstrated significant effects on hair growth, particularly in human hair follicles (HFs). Research indicates that spermidine promotes hair shaft elongation and prolongs the anagen phase (the active growth phase of hair).
Case Study:
- In a controlled study, treatment with spermidine at a concentration of 0.5 µM resulted in over a 20% increase in hair shaft production after six days of culture .
- The treatment also upregulated keratin 15 (K15) and keratin 19 (K19), markers associated with hair follicle epithelial stem cells, suggesting spermidine's role in enhancing stem cell activity within HFs .
Neuroprotective Effects
Spermidine has shown promise in neuroprotection, particularly in models of neurodegenerative diseases. Its antioxidant properties help reduce oxidative stress, which is implicated in conditions like multiple sclerosis.
Research Findings:
- A study involving murine models of experimental autoimmune encephalomyelitis (EAE) revealed that spermidine treatment significantly reduced clinical symptoms and demyelination .
- The treatment improved visual function and reduced retinal ganglion cell apoptosis induced by oxidative stress, highlighting its potential as a therapeutic agent for neurodegenerative diseases .
Emerging studies suggest that spermidine may play a role in cancer biology. Urinary polyamines, including spermidine, have been investigated as potential biomarkers for colorectal cancer (CRC) detection.
Key Insights:
Mechanism of Action
- Spermidine influences various molecular targets and pathways.
- It induces autophagy, enhances antioxidant defenses, and modulates gene expression.
- The exact mechanisms are complex and context-dependent.
Comparison with Similar Compounds
Similar Compounds: Other polyamines like spermine and putrescine share similarities.
Uniqueness: Spermidine-d6’s deuterium labeling distinguishes it from natural spermidine.
Biological Activity
Spermidine-d6 is a deuterated form of spermidine, a biogenic polyamine known for its significant biological roles, particularly in cellular growth, differentiation, and longevity. This article explores the biological activity of this compound, focusing on its mechanisms of action, effects on various cell types, and potential therapeutic applications.
Overview of this compound
Spermidine is involved in numerous cellular processes, including autophagy, cell proliferation, and apoptosis. The introduction of deuterium in this compound allows for enhanced tracking in metabolic studies and pharmacokinetic evaluations. This compound has gained attention for its potential applications in aging research and cancer therapy.
Spermidine and its derivatives exert their effects through several mechanisms:
- Autophagy Induction : Spermidine promotes autophagy by inhibiting histone deacetylases (HDACs), which enhances the acetylation of proteins involved in autophagic processes. This mechanism is crucial for maintaining cellular homeostasis and combating age-related diseases .
- Mitochondrial Function : Research indicates that spermidine enhances mitochondrial function by activating mitochondrial trifunctional protein (MTP), which plays a role in fatty acid oxidation. This activation is vital for energy production and cellular metabolism .
- Cell Cycle Regulation : Spermidine influences the cell cycle by modulating the expression of key regulatory proteins. It has been shown to inhibit tumor cell proliferation by interfering with the cell cycle progression .
1. Hair Growth Stimulation
Spermidine has been documented to stimulate hair growth significantly. In a study involving human hair follicles, treatment with spermidine at a concentration of 0.5 µM resulted in over a 20% increase in hair shaft production after six days. This effect was attributed to the upregulation of keratin markers such as K15 and K19, which are indicative of epithelial progenitor cells in hair follicles .
2. Cancer Therapy
Spermidine shows promise as an anticancer agent. It has been observed to enhance the efficacy of immunotherapy treatments by rejuvenating aged immune cells, particularly CD8+ T cells. In vivo studies demonstrated that spermidine supplementation improved anti-tumor activity when combined with PD-1 blockade therapies . Moreover, spermidine's ability to induce apoptosis in cancer cells has been linked to its modulation of autophagy pathways .
3. Antioxidant Properties
Spermidine exhibits antioxidant effects by regulating oxidative stress responses through the Keap1-Nrf2 pathway. This regulation leads to increased expression of antioxidant enzymes that protect cells from damage caused by reactive oxygen species (ROS) . Additionally, spermidine has been shown to protect ovarian function by reducing follicular atresia and enhancing antioxidant enzyme activity .
Pharmacokinetics
Recent studies have explored the pharmacokinetics of this compound. A randomized controlled trial indicated that oral administration of spermidine leads to increased levels of spermine in plasma but does not significantly alter spermidine or putrescine levels . These findings suggest that dietary spermidine may be metabolized into spermine prior to systemic circulation.
Case Studies
- Spermidine in Aging Research : A study highlighted that lifelong oral administration of spermidine extended lifespan in mice by approximately 25%, primarily through autophagy activation mediated by MAP1S .
- Spermidine and SARS-CoV-2 : Research indicated that spermidine could inhibit SARS-CoV-2 replication significantly when administered prior to infection, suggesting potential antiviral properties against COVID-19 .
Summary Table of Biological Activities
Properties
IUPAC Name |
N'-(3-amino-1,1,2,2,3,3-hexadeuteriopropyl)butane-1,4-diamine | |
|---|---|---|
| Details | Computed by LexiChem 2.6.6 (PubChem release 2019.06.18) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C7H19N3/c8-4-1-2-6-10-7-3-5-9/h10H,1-9H2/i3D2,5D2,7D2 | |
| Details | Computed by InChI 1.0.5 (PubChem release 2019.06.18) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
ATHGHQPFGPMSJY-RCKJUGKUSA-N | |
| Details | Computed by InChI 1.0.5 (PubChem release 2019.06.18) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C(CCNCCCN)CN | |
| Details | Computed by OEChem 2.1.5 (PubChem release 2019.06.18) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
[2H]C([2H])(C([2H])([2H])N)C([2H])([2H])NCCCCN | |
| Details | Computed by OEChem 2.1.5 (PubChem release 2019.06.18) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C7H19N3 | |
| Details | Computed by PubChem 2.1 (PubChem release 2019.06.18) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Weight |
151.28 g/mol | |
| Details | Computed by PubChem 2.1 (PubChem release 2021.05.07) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Synthesis routes and methods I
Procedure details





Synthesis routes and methods II
Procedure details





Synthesis routes and methods III
Procedure details





Synthesis routes and methods IV
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.














