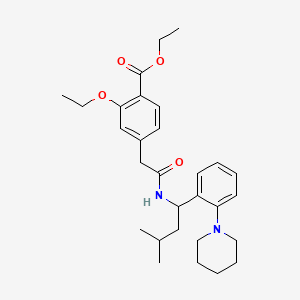
Repaglinide ethyl ester
Overview
Description
Repaglinide ethyl ester is a chemical compound that serves as an intermediate in the synthesis of repaglinide, a medication used to manage type 2 diabetes mellitus. Repaglinide is a non-sulfonylurea insulin secretagogue that helps regulate blood glucose levels by stimulating insulin release from pancreatic beta cells .
Preparation Methods
Synthetic Routes and Reaction Conditions
Repaglinide ethyl ester can be synthesized through various synthetic routes. One common method involves the esterification of 3-hydroxyphenylacetic acid, followed by formylation, oxidation, etherification, and selective hydrolysis . The reaction conditions, such as temperature, time, solvent, and substrate ratios, are optimized to achieve high yields and purity .
Industrial Production Methods
In industrial settings, the production of this compound involves large-scale synthesis using similar reaction steps. The process is designed to be scalable and environmentally friendly, minimizing waste and optimizing resource utilization .
Chemical Reactions Analysis
Types of Reactions
Repaglinide ethyl ester undergoes various chemical reactions, including:
Oxidation: Conversion of functional groups to higher oxidation states.
Reduction: Conversion of functional groups to lower oxidation states.
Substitution: Replacement of one functional group with another.
Common Reagents and Conditions
Common reagents used in these reactions include oxidizing agents like potassium permanganate, reducing agents like lithium aluminum hydride, and substitution reagents like ethyl bromide . The reactions are typically carried out under controlled conditions to ensure high selectivity and yield.
Major Products Formed
The major products formed from these reactions include various intermediates that are further processed to produce repaglinide .
Scientific Research Applications
Chemical Properties and Mechanism of Action
Repaglinide ethyl ester has the molecular formula C29H40N2O4 and a molecular weight of approximately 480.649 g/mol. It functions as a rapid-acting insulin secretagogue, stimulating insulin release from pancreatic beta cells. The compound primarily acts by binding to the sulfonylurea receptor, which facilitates insulin secretion in response to glucose levels.
Scientific Research Applications
This compound finds utility in several scientific domains:
Pharmaceutical Development
This compound serves as an intermediate in the synthesis of repaglinide and other related compounds. Its unique structural properties enhance solubility and bioavailability, making it a valuable component in drug formulation aimed at improving therapeutic efficacy against diabetes.
Analytical Chemistry
The compound is utilized in various analytical methods, including high-performance liquid chromatography (HPLC), for the separation and quantification of repaglinide in biological samples. This application is crucial for pharmacokinetic studies and quality control during pharmaceutical production.
Transdermal Drug Delivery Systems
Research has explored the formulation of transdermal patches using this compound to provide controlled drug release. Studies indicate that these systems can enhance patient compliance by offering an alternative to oral administration.
Biological Studies
This compound is studied for its interactions with various biological targets, particularly regarding its role in insulin secretion and glucose metabolism. Investigations into its effects on cellular mechanisms have provided insights into potential combination therapies for diabetes management.
Case Studies
Recent clinical trials have underscored the effectiveness of this compound in diverse populations:
- Type 2 Diabetes Patients : A randomized controlled trial demonstrated significant reductions in both fasting and postprandial glucose levels among patients treated with this compound compared to placebo.
- Elderly Population : Another study focused on elderly patients indicated that this compound was effective with a favorable safety profile, showing minimal adverse effects.
Mechanism of Action
Repaglinide ethyl ester itself does not have a direct mechanism of action, as it is an intermediate compound. repaglinide, the final product, exerts its effects by inhibiting ATP-sensitive potassium channels in pancreatic beta cells. This inhibition leads to membrane depolarization, opening of calcium channels, and subsequent insulin release .
Comparison with Similar Compounds
Similar Compounds
Similar compounds to repaglinide ethyl ester include:
Nateglinide: Another meglitinide class drug used to manage type 2 diabetes.
Mitiglinide: A similar insulin secretagogue with a different chemical structure.
Uniqueness
This compound is unique due to its specific role as an intermediate in the synthesis of repaglinide. Its optimized synthetic route and reaction conditions make it a valuable compound in the pharmaceutical industry .
Properties
Molecular Formula |
C29H40N2O4 |
|---|---|
Molecular Weight |
480.6 g/mol |
IUPAC Name |
ethyl 2-ethoxy-4-[2-[[3-methyl-1-(2-piperidin-1-ylphenyl)butyl]amino]-2-oxoethyl]benzoate |
InChI |
InChI=1S/C29H40N2O4/c1-5-34-27-19-22(14-15-24(27)29(33)35-6-2)20-28(32)30-25(18-21(3)4)23-12-8-9-13-26(23)31-16-10-7-11-17-31/h8-9,12-15,19,21,25H,5-7,10-11,16-18,20H2,1-4H3,(H,30,32) |
InChI Key |
FTCMVLQJMIXDSI-UHFFFAOYSA-N |
Canonical SMILES |
CCOC1=C(C=CC(=C1)CC(=O)NC(CC(C)C)C2=CC=CC=C2N3CCCCC3)C(=O)OCC |
Origin of Product |
United States |
Synthesis routes and methods I
Procedure details





Synthesis routes and methods II
Procedure details








Synthesis routes and methods III
Procedure details




Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.













