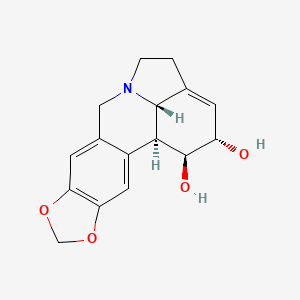
Lycorine
Overview
Description
Preparation Methods
Synthetic Routes and Reaction Conditions
Lycorine can be synthesized through several methods. One notable synthetic route involves the use of cyclopropyl acyliminium ion expansion and rearrangement, followed by a Diels-Alder reaction to construct the key diene intermediate . This method, developed by Boeckman et al., is considered one of the most elegant synthetic routes for this compound .
Industrial Production Methods
Industrial production of this compound typically involves extraction from plant sources. Air-dried powdered bulbs of plants like Sternbergia fischeriana are used to isolate this compound . Advanced techniques such as in vitro plant cell suspension cultures and bioreactors are also employed to produce this compound sustainably .
Chemical Reactions Analysis
Types of Reactions
Lycorine undergoes various chemical reactions, including oxidation, reduction, and substitution. It is known to inhibit protein synthesis and may inhibit ascorbic acid biosynthesis .
Common Reagents and Conditions
Common reagents used in this compound reactions include oxidizing agents for oxidation reactions and reducing agents for reduction reactions. Specific conditions vary depending on the desired reaction and product.
Major Products Formed
Major products formed from this compound reactions include derivatives with enhanced biological activities. For example, dihydrothis compound, generated through the hydrogenation of this compound, has been used clinically due to its better resistance against amebic dysentery and lower toxicity .
Scientific Research Applications
Lycorine is a naturally occurring alkaloid that has garnered attention for its diverse range of pharmacological activities, particularly in the realm of cancer research. Studies have explored its potential as an anti-cancer agent, demonstrating its effects on various cancer cell lines and tumor models .
Scientific Research Applications
Anti-Cancer Activity: this compound has demonstrated anticancer activities in several studies . It exhibits selective cytotoxicity effects on leukemia, cervical cancer, and multiple myeloma . Research indicates that this compound can decrease proliferation, migration, invasion, and survival of prostate cancer cell lines . In vivo experiments have shown that this compound reduces both the weight and volume of prostate cancer xenografts and inhibits cancer growth and metastasis in multiple organs, improving survival rates in mice .
Mechanism of Action in Cancer Cells: this compound's anti-cancer effects are linked to its ability to interfere with key signaling pathways involved in cancer development and progression . It inhibits the activation of EGF-induced JAK/STAT signaling and multiple STAT3 downstream targets, such as cyclin D1, Bcl-2, Bcl-xL, matrix metalloproteinase 2 (MMP2), and the EMT promoter Twist . In colorectal cancer cells, this compound may induce cell cycle arrest and exert cytostatic effects by activating ROS/p38 and AKT signaling pathways . It can also downregulate the protein expression levels of MMP-2, MMP-7, and MMP-9, and reverse the EMT process in CRC cells .
Effects on Cell Cycle Arrest: this compound can induce cell cycle arrest in cancer cells . In HCT116 cells, this compound treatment results in a significantly higher proportion of cells in the G2/M phase, while in LoVo cells, it leads to a significantly higher ratio of cells in the S and G2/M phases . this compound also downregulated the protein expression levels of cyclin D1 and cyclin E1, and increased p21 and Smad4 protein expression levels .
Other Pharmacological Effects: this compound possesses strong pharmacological effects on many diseases, including anti-leukemia, anti-tumor, anti-angiogenesis, anti-virus, and anti-bacteria . It has a strong anti-acetylcholinesterase effect .
Safety in the Central Nervous System: In vitro studies suggest that this compound regulates immune cells, indicating its involvement as a cellular mechanism underlying the suppression of pain .
Tumor Xenograft Studies: this compound is effective against tumor xenografts and has effectively inhibited tumor growth in B16F10 melanoma-bearing mice, HL-60 xenografted SCID mice, ovarian cancer Hey1B bearing nude mice, and multiple myeloma (MM) cell xenografted NOD/SCID mice .
Tables
Table 1: this compound's Effects on Prostate Cancer Cell Lines (in vitro)
Table 2: this compound's Impact on Colorectal Cancer (CRC) Cell Lines (in vitro)
Case Studies
Prostate Cancer Xenograft Model: In vivo experiments using a PC-3M-luc orthotopic xenograft model demonstrated that this compound inhibits prostate cancer growth and metastasis . Intraperitoneal administration of this compound reduced both the weight and volume of ectopically PC-3M subcutaneous xenografts by approximately 80% without causing obvious toxicity .
Colorectal Cancer Cell Lines: this compound inhibited cell proliferation in HCT116, LoVo, and SW480 cells, with IC50 values of 1.4, 3.8, and 1.3 µmol/l, respectively, following treatment for 72 hours .
Mechanism of Action
Lycorine exerts its effects through multiple mechanisms. It inhibits protein synthesis and may inhibit ascorbic acid biosynthesis . In cancer cells, this compound induces apoptosis and autophagy by targeting pathways such as the TCRP1/Akt/mTOR axis . It also inhibits angiogenesis by docking to PDGFRα, thereby restraining its activity .
Comparison with Similar Compounds
Lycorine is unique among alkaloids due to its rigid ring skeleton and contiguous chiral centers . Similar compounds include:
Galanthamine: Used in the treatment of Alzheimer’s disease.
Lycoramine: Used for treating post-polio syndrome.
Dihydrothis compound: Used clinically for its lower toxicity and resistance against amebic dysentery.
These compounds share structural similarities with this compound but have distinct pharmacological properties and applications.
Biological Activity
Lycorine is a natural alkaloid derived from various plants, particularly those in the Amaryllidaceae family. It has garnered significant attention due to its diverse biological activities, including anti-cancer, antiviral, and neuroprotective properties. This article provides a comprehensive overview of the biological activity of this compound, supported by research findings and data tables.
This compound's chemical structure is characterized by a benzylphenethylamine backbone, which is crucial for its biological activity. The compound exhibits multiple mechanisms of action, primarily through the inhibition of various cellular pathways associated with cancer and viral infections.
- Inhibition of DNA Topoisomerase I : this compound has been shown to inhibit DNA topoisomerase I, an enzyme critical for DNA replication in cancer cells. This inhibition leads to cytostatic effects rather than cytotoxicity, making it a potential candidate for cancer therapy .
- Autophagy-Associated Apoptosis : Research indicates that this compound can induce autophagy-associated apoptosis by targeting MEK2, a key regulator in cancer signaling pathways. This mechanism enhances the therapeutic efficacy when combined with other agents like vemurafenib in colorectal cancer models .
- Cell Cycle Arrest : this compound has been found to induce cell cycle arrest in various cancer cell lines, specifically at the G2/M phase, thereby inhibiting cell proliferation and migration .
Anticancer Activity
This compound exhibits significant anticancer properties across various cancer types:
- Breast and Prostate Cancer : It promotes LRP6 phosphorylation and Wnt signaling transduction, which are beneficial against breast and prostate cancers .
- Bladder Cancer : In bladder cancer cell lines, this compound enhances sensitivity to gemcitabine, a common chemotherapeutic agent .
- Leukemia : Studies have demonstrated that this compound suppresses tumor growth in leukemia models (HL-60 cell line) through apoptosis induction .
Antiviral Activity
This compound has been identified as an effective inhibitor of Hepatitis C Virus (HCV), acting by downregulating Hsc70 expression in host cells. However, its cytotoxicity limits its clinical application as an antiviral agent .
Neuroprotective Effects
This compound exhibits analgesic properties superior to common analgesics like aspirin and indomethacin. It also shows anticonvulsant effects in animal models, indicating potential use in treating neurological disorders .
Table 1: Summary of Biological Activities of this compound
Case Studies
- Colorectal Cancer Study : A study demonstrated that this compound treatment led to significant apoptosis in HCT116-derived xenografts, with enhanced effects observed when combined with vemurafenib .
- Bladder Cancer Sensitivity : In T24 and 5637 bladder cancer cells, this compound increased gemcitabine sensitivity by approximately 40-60%, indicating its potential as an adjunct therapy .
- Cytotoxicity Studies : Structure-activity relationship studies revealed that modifications to this compound's structure could reduce its cytotoxicity while maintaining antiviral efficacy against HCV .
Q & A
Basic Research Questions
Q. What experimental approaches are commonly used to investigate Lycorine's anticancer mechanisms?
Researchers employ:
- NF-κB reporter gene assays to monitor constitutive and cytokine-induced pathway activity in prostate cancer cells (PC3/DU145) .
- Transwell migration assays and wound healing assays to quantify inhibition of hepatocellular carcinoma (HCC) cell motility .
- Flow cytometry with annexin V/PI staining to assess apoptosis induction in colorectal cancer (CRC) cells .
- Western blotting for key proteins (e.g., cyclins, Bcl-2, caspase-3) to validate cell cycle arrest (G2/M phase) and apoptotic pathways .
Q. How is this compound's effect on cell cycle progression evaluated in cancer models?
- Flow cytometry with propidium iodide staining quantifies cell cycle phase distribution (e.g., G2/M arrest in HepG2 cells) .
- ModFit LT software analyzes cyclin A, cyclin B1, and cdc2 expression changes, correlating with G2/M checkpoint dysregulation .
Q. What molecular targets of this compound have been identified in leukemia research?
- p21 upregulation via p53-independent pathways in HL-60 cells, confirmed via siRNA knockdown to link p21 to apoptosis .
- HDAC activity assays using colorimetric kits reveal cell-line-specific effects (e.g., reduced activity in K562 but not HL-60 cells) .
Q. Which in vitro models are standard for studying this compound's antiviral activity?
- MDCK cells infected with H5N1 influenza assess viral replication via plaque assays and proteomic profiling .
- Time-of-addition experiments determine optimal this compound treatment windows to inhibit nuclear export of viral ribonucleoproteins (vRNPs) .
Advanced Research Questions
Q. How can researchers resolve contradictions in this compound's effects on HDAC activity across cell lines?
- Perform cell-type-specific HDAC activity profiling using colorimetric assays (e.g., unaffected in HL-60 vs. inhibited in K562) .
- Combine RNAi-mediated gene silencing with this compound treatment to confirm context-dependent targets (e.g., p21 vs. HDACs) .
Q. What strategies integrate proteomic data into this compound mechanism studies?
- Tandem mass tag (TMT)-based quantitative proteomics identifies differentially expressed proteins (e.g., Nup93 downregulation in H5N1-infected cells) .
- GO enrichment and pathway analysis link proteomic changes to biological processes (e.g., nuclear-cytoplasmic transport inhibition) .
Q. How do conflicting results on this compound's modulation of NF-κB vs. STAT pathways inform experimental design?
- Compare constitutive vs. cytokine-induced signaling in prostate cancer models (e.g., NF-κB inhibition in PC3 vs. STAT3 suppression in PC-3M-luc) .
- Use dual-luciferase reporter systems and phospho-specific antibodies to dissect cross-talk between pathways .
Q. What in vivo models best capture this compound's antitumor and antimetastatic effects?
- Orthotopic xenografts (e.g., RM-1 prostate cancer) monitor metastasis to liver, lung, and bone via bioluminescence imaging .
- Immunohistochemistry validates pathway inhibition (e.g., reduced Ki-67 and phospho-p65 in tumor tissues) .
Q. How can researchers distinguish this compound-induced autophagy from apoptosis?
- Tandem mRFP-GFP-LC3 transfection distinguishes autophagosome formation (yellow puncta) vs. lysosomal degradation (red puncta) .
- JC-1 mitochondrial membrane potential assays and TEM imaging confirm apoptosis-associated mitochondrial damage .
Q. What methodologies validate this compound's synergy with existing therapies (e.g., cisplatin)?
- Combination index (CI) analysis using Chou-Talalay method quantifies synergism in cervical cancer models (e.g., reduced CT45A1-YAP signaling enhances cisplatin sensitivity) .
- Orthotopic co-treatment studies assess tumor regression and metastasis in vivo .
Q. Key Considerations for Experimental Design
- Dose-response validation : Use this compound concentrations ≤20 µM to avoid off-target cytotoxicity .
- Cell line selection : Prioritize p53-null models (e.g., HL-60, PC3) to isolate p53-independent mechanisms .
- Data reproducibility : Include pharmacopeial reference standards (e.g., USP/EP) for analytical method validation .
Properties
IUPAC Name |
(1S,17S,18S,19S)-5,7-dioxa-12-azapentacyclo[10.6.1.02,10.04,8.015,19]nonadeca-2,4(8),9,15-tetraene-17,18-diol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C16H17NO4/c18-11-3-8-1-2-17-6-9-4-12-13(21-7-20-12)5-10(9)14(15(8)17)16(11)19/h3-5,11,14-16,18-19H,1-2,6-7H2/t11-,14-,15+,16+/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
XGVJWXAYKUHDOO-DANNLKNASA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1CN2CC3=CC4=C(C=C3C5C2C1=CC(C5O)O)OCO4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C1CN2CC3=CC4=C(C=C3[C@H]5[C@H]2C1=C[C@@H]([C@H]5O)O)OCO4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C16H17NO4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID60197208 | |
| Record name | Lycorine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID60197208 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
287.31 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
476-28-8 | |
| Record name | Lycorine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=476-28-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Lycorine | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000476288 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | lycorine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=683873 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | lycorine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=401360 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Lycorine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID60197208 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Lycorine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.006.822 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | LYCORINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/I9Q105R5BU | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.















