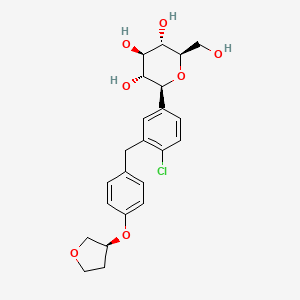
Empagliflozin
Overview
Description
Empagliflozin is an oral medication used primarily to manage type 2 diabetes mellitus. It belongs to the class of drugs known as sodium-glucose co-transporter-2 (SGLT2) inhibitors. By inhibiting SGLT2, this compound reduces glucose reabsorption in the kidneys, leading to increased glucose excretion through urine. This helps lower blood sugar levels in patients with type 2 diabetes .
Preparation Methods
Synthetic Routes and Reaction Conditions: Empagliflozin is synthesized through a multi-step process involving several key intermediates. One common synthetic route involves the following steps:
Formation of Intermediate I: The initial step involves the reaction of 4-chloro-3-(4-(tetrahydrofuran-3-yloxy)benzyl)phenyl with a suitable glucosyl donor to form Intermediate I.
Formation of Intermediate II: Intermediate I undergoes further reactions, including protection and deprotection steps, to yield Intermediate II.
Final Coupling: Intermediate II is then coupled with a suitable glucosyl donor under basic conditions to form this compound
Industrial Production Methods: Industrial production of this compound typically involves large-scale synthesis using optimized reaction conditions to ensure high yield and purity. The process includes:
Refluxing: The reaction mixture is refluxed to facilitate the formation of the desired product.
Purification: The crude product is purified using techniques such as recrystallization and chromatography to obtain this compound with high purity
Chemical Reactions Analysis
Types of Reactions: Empagliflozin undergoes various chemical reactions, including:
Hydrolysis: The compound is susceptible to hydrolysis, especially under acidic and basic conditions.
Common Reagents and Conditions:
Oxidative Degradation: Reagents such as hydrogen peroxide or other oxidizing agents can induce oxidative degradation.
Hydrolysis: Acidic or basic conditions, such as hydrochloric acid or sodium hydroxide, can facilitate hydrolysis.
Major Products Formed:
Oxidative Degradation Products: Various oxidation products can be formed, depending on the specific conditions and reagents used.
Hydrolysis Products: Hydrolysis can lead to the formation of smaller fragments and degradation products.
Scientific Research Applications
Empagliflozin is a relatively new drug that inhibits the sodium–glucose cotransporter 2 (SGLT2), increasing urinary glucose excretion . Originally used to induce a hypoglycemic effect in patients with type 2 diabetes mellitus (T2DM), this compound has demonstrated other beneficial effects, including nephroprotection and as a breakthrough in treating heart failure (HF) . Studies have shown benefits in patients with and without T2DM .
Scientific Research Applications
A study evaluated this compound initiated in-hospital for acute heart failure . The primary outcome was clinical benefit, assessed using a hierarchical composite of death from any cause, the number of heart failure events, time to first heart failure event, or a 5 point or greater difference in change from baseline in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score at 90 days . More patients treated with this compound had clinical benefit compared with placebo . Clinical benefit was seen for both acute de novo and decompensated chronic heart failure and regardless of ejection fraction or diabetes status . this compound was well tolerated, with fewer serious adverse events than placebo . These results extend and complement those of EMPEROR-Reduced and EMPEROR-Preserved by focusing on patients hospitalized for acute heart failure across the range of ejection fraction .
Renal Protection: this compound significantly reduces the relative risk of developing or worsening nephropathy by 39% compared to placebo . The relative risk reduction for progression to macroalbuminuria between the this compound and placebo groups was 38% . The risk of doubling plasma creatinine levels was reduced by 44% in the this compound group, and the relative risk of starting renal replacement therapy (RRT) was significantly 55% lower .
Alzheimer's Disease: A five-drug combination consisting of Tofacitinib, Niraparib, Baricitinib, this compound, and Doxercalciferol can serve as a promising drug combination for AD treatment . this compound, an SGLT2 inhibitor, regulates the insulin signaling pathway linking diabetes and AD . this compound can target EGFR in the MAPK, FOXO, and Rap1 signaling pathways via SLC5A1 .
Improved Cardiac Function: this compound administration to nondiabetic HFrEF patients significantly improves LV volumes, LV mass, LV systolic function, functional capacity, and quality . this compound significantly ameliorated adverse LV remodeling, decreased LV volumes and LV hypertrophy, reduced neurohormonal activation, and improved cardiac systolic function compared with the control group . Moreover, this compound also improved diastolic function in this HFrEF model .
Improved Clinical Stability: this compound reduces the risk of cardiovascular death or hospitalization for heart failure in patients with heart failure and a reduced ejection fraction . this compound reduced the total number of heart failure hospitalizations that required intensive care and that required a vasopressor or positive inotropic drug or mechanical or surgical intervention . Patients assigned to this compound were 20% to 40% more likely to experience an improvement in New York Heart Association functional class and were 20% to 40% less likely to experience worsening of New York Heart Association functional class, with statistically significant effects that were apparent 28 days after randomization and maintained during long-term follow-up .
Data Table
Mechanism of Action
Empagliflozin exerts its effects by inhibiting the sodium-glucose co-transporter-2 (SGLT2) in the kidneys. This inhibition reduces the reabsorption of glucose from the glomerular filtrate, leading to increased glucose excretion through urine. The reduction in blood glucose levels helps manage type 2 diabetes. Additionally, this compound has been shown to have cardioprotective and nephroprotective effects, which are thought to be mediated through mechanisms such as reducing oxidative stress and improving endothelial function .
Comparison with Similar Compounds
Empagliflozin is part of the SGLT2 inhibitor class of drugs. Other similar compounds in this class include:
Canagliflozin: Another SGLT2 inhibitor used to manage type 2 diabetes.
Dapagliflozin: This compound is also used to treat type 2 diabetes and has been approved for use in heart failure.
Ertugliflozin: Another SGLT2 inhibitor with similar mechanisms of action but different pharmacokinetic properties.
Uniqueness of this compound: this compound is unique in its high selectivity for SGLT2 over SGLT1, which contributes to its efficacy and safety profile. It has also been shown to have significant cardiovascular and renal benefits, making it a valuable option for patients with type 2 diabetes and comorbid conditions .
Biological Activity
Empagliflozin is a sodium-glucose cotransporter 2 (SGLT2) inhibitor primarily used in the management of type 2 diabetes mellitus (T2DM). Its biological activity extends beyond glycemic control, showing significant cardiovascular benefits and effects on cardiac metabolism. This article reviews the biological activity of this compound, supported by recent studies, clinical trials, and case studies.
This compound works by inhibiting the SGLT2 protein in the kidneys, leading to reduced glucose reabsorption and increased glucose excretion. This mechanism not only lowers blood glucose levels but also has secondary effects on cardiovascular health.
- Cardiac Energy Metabolism : Recent studies indicate that this compound enhances cardiac energy status by increasing ATP levels in both cytosolic and mitochondrial compartments within cardiomyocytes. In a study involving db/db mice, this compound treatment resulted in a significant increase in mitochondrial ATP levels, which was associated with improved cardiac function during ischemic conditions .
- Cardioprotection : this compound has been shown to provide cardioprotective effects, particularly in models of heart failure. It improves recovery capacity in ischemic-reperfusion scenarios and maintains ATP levels during stress conditions .
Clinical Evidence
This compound's efficacy has been validated through various clinical trials:
- EMPULSE Trial : This double-blind trial evaluated this compound in patients hospitalized for acute heart failure. Results indicated that this compound significantly improved clinical outcomes compared to placebo, with a stratified win ratio of 1.36 (p = 0.0054), demonstrating its potential benefits even when initiated during hospitalization .
- Long-term Cardiovascular Outcomes : In patients with chronic heart failure, this compound reduced the risk of cardiovascular death and heart failure hospitalization. The drug was well tolerated, with serious adverse events occurring less frequently than in the placebo group .
Case Studies
Several case studies highlight the biological activity of this compound:
- Case Study on Cardiac Remodeling : A study focused on patients with T2DM and coronary artery disease found that this compound treatment led to a reduction in left ventricular mass over time, suggesting beneficial remodeling effects on the heart .
- Impact on Diabetic Patients : Research involving diabetic patients showed that this compound effectively reduced fasting plasma glucose and glycated hemoglobin levels while also improving markers of cardiovascular health, such as blood pressure and weight management .
Summary of Findings
The following table summarizes key findings from recent studies regarding the biological activity of this compound:
Properties
IUPAC Name |
(2S,3R,4R,5S,6R)-2-[4-chloro-3-[[4-[(3S)-oxolan-3-yl]oxyphenyl]methyl]phenyl]-6-(hydroxymethyl)oxane-3,4,5-triol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C23H27ClO7/c24-18-6-3-14(23-22(28)21(27)20(26)19(11-25)31-23)10-15(18)9-13-1-4-16(5-2-13)30-17-7-8-29-12-17/h1-6,10,17,19-23,25-28H,7-9,11-12H2/t17-,19+,20+,21-,22+,23-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
OBWASQILIWPZMG-QZMOQZSNSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1COCC1OC2=CC=C(C=C2)CC3=C(C=CC(=C3)C4C(C(C(C(O4)CO)O)O)O)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C1COC[C@H]1OC2=CC=C(C=C2)CC3=C(C=CC(=C3)[C@H]4[C@@H]([C@H]([C@@H]([C@H](O4)CO)O)O)O)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C23H27ClO7 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID601026093 | |
| Record name | Empagliflozin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID601026093 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
450.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
The vast majority of glucose filtered through the glomerulus is reabsorbed within the proximal tubule, primarily via SGLT2 (sodium-glucose linked co-transporter-2) which is responsible for ~90% of the total glucose reabsorption within the kidneys. Na+/K+-ATPase on the basolateral membrane of proximal tubular cells utilize ATP to actively pump Na+ ions into the interstitium surrounding the tubule, establishing a Na+ gradient within the tubular cell. SGLT2 on the apical membrane of these cells then utilize this gradient to facilitate secondary active co-transport of both Na+ and glucose out of the filtrate, thereby reabsorbing glucose back into the blood – inhibiting this co-transport, then, allows for a marked increase in glucosuria and decrease in blood glucose levels. Empagliflozin is a potent inhibitor of renal SGLT2 transporters located in the proximal tubules of the kidneys and works to lower blood glucose levels via an increase in glucosuria. Empagliflozin also appears to exert cardiovascular benefits - specifically in the prevention of heart failure - independent of its blood glucose-lowering effects, though the exact mechanism of this benefit is not precisely understood. Several theories have been posited, including the potential inhibition of Na+/H+ exchanger (NHE) 1 in the myocardium and NHE3 in the proximal tubule, reduction of pre-load via diuretic/natriuretic effects and reduction of blood pressure, prevention of cardiac fibrosis via suppression of pro-fibrotic markers, and reduction of pro-inflammatory adipokines. | |
| Record name | Empagliflozin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09038 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
864070-44-0 | |
| Record name | Empagliflozin | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=864070-44-0 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Empagliflozin [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0864070440 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Empagliflozin | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09038 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Empagliflozin | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID601026093 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | (2S,3R,4R,5S,6R)-2-(4-chloro-3-(4-((S)-tetrahydrofuran-3-yloxy)benzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | EMPAGLIFLOZIN | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/HDC1R2M35U | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.















