Cabotegravir
Overview
Description
Cabotegravir is an antiretroviral medication used for the treatment and prevention of HIV/AIDS. It is an integrase strand transfer inhibitor (INSTI) that prevents the integration of the viral genome into the host DNA, thereby inhibiting viral replication . This compound is available in both oral and long-acting injectable forms, making it a versatile option for HIV treatment and pre-exposure prophylaxis (PrEP) .
Preparation Methods
Synthetic Routes and Reaction Conditions
The synthesis of cabotegravir involves multiple steps, starting from commercially available starting materialsThe reaction conditions typically involve the use of strong bases, such as sodium hydride, and various organic solvents .
Industrial Production Methods
Industrial production of this compound follows similar synthetic routes but on a larger scale. The process is optimized for yield and purity, with stringent quality control measures in place. The final product is formulated into tablets or injectable suspensions, depending on the intended use .
Chemical Reactions Analysis
Types of Reactions
Cabotegravir undergoes several types of chemical reactions, including:
Oxidation: this compound can be oxidized to form various metabolites.
Reduction: Reduction reactions can modify the functional groups on the this compound molecule.
Substitution: Substitution reactions can introduce different functional groups into the this compound structure.
Common Reagents and Conditions
Common reagents used in these reactions include oxidizing agents like hydrogen peroxide, reducing agents like sodium borohydride, and various organic solvents. The reaction conditions are typically controlled to ensure the desired product is obtained with high purity .
Major Products Formed
The major products formed from these reactions include various metabolites and derivatives of this compound, which can have different pharmacological properties .
Scientific Research Applications
Applications in HIV Treatment
-
Long-Acting Injectable Regimen :
- Cabotegravir has been combined with rilpivirine in a long-acting injectable formulation. This regimen has shown high efficacy in maintaining viral suppression in individuals living with HIV, demonstrating a strong barrier to resistance .
- Clinical trials indicate that this two-drug regimen is effective in antiretroviral-naive patients, with ongoing studies assessing its safety and efficacy over extended periods .
- Oral Administration :
Applications in Pre-Exposure Prophylaxis (PrEP)
-
Efficacy in HIV Prevention :
- Long-acting injectable this compound has demonstrated superior efficacy compared to daily oral tenofovir disoproxil fumarate plus emtricitabine in preventing HIV infection among high-risk populations, including men who have sex with men and transgender women .
- The HPTN 083 study highlighted that this compound maintained high levels of protection against HIV over time, with minimal instances of breakthrough infections linked to integrase strand transfer inhibitor resistance .
-
Adherence and Acceptance :
- The convenience of fewer injections compared to daily pills enhances adherence among users. Studies have indicated that individuals prefer long-acting formulations due to reduced daily burden and improved lifestyle integration .
- Real-world data suggest that adherence rates remain high with this compound injections, supporting its role as a preferred option for PrEP .
Case Study 1: Implementation in Diverse Populations
A recent report explored the implementation pathways for this compound among various populations at risk of HIV. The findings underscored the importance of tailoring delivery methods to meet the needs of individuals with different health backgrounds, including those with mental health challenges or substance use disorders .
Case Study 2: Resistance Patterns
A case series involving patients with NNRTI resistance demonstrated that this compound combined with lenacapavir resulted in high rates of virologic suppression (94%). This highlights this compound's effectiveness even in populations facing significant treatment challenges due to resistance .
Summary of Findings
| Application | Formulation | Efficacy | Adherence |
|---|---|---|---|
| Treatment of HIV | Long-acting injectable | High viral suppression | Monthly/quarterly dosing |
| Pre-exposure prophylaxis (PrEP) | Injectable & oral | Superior efficacy over daily oral PrEP | High adherence rates |
Mechanism of Action
Cabotegravir exerts its effects by binding to the active site of the HIV integrase enzyme. This prevents the strand transfer of the viral genome into the host DNA, thereby inhibiting viral replication . The molecular targets include the integrase enzyme and the viral DNA, and the pathways involved are related to the viral replication cycle .
Comparison with Similar Compounds
Similar Compounds
Dolutegravir: Another integrase inhibitor with a similar mechanism of action.
Raltegravir: The first integrase inhibitor approved for HIV treatment.
Elvitegravir: An integrase inhibitor used in combination with other antiretroviral drugs.
Uniqueness of Cabotegravir
This compound is unique due to its long-acting injectable formulation, which provides sustained drug levels and reduces the need for daily dosing. This makes it particularly useful for individuals who have difficulty adhering to daily medication regimens .
Biological Activity
Cabotegravir, an integrase strand transfer inhibitor (INSTI), is primarily recognized for its role in the prevention and treatment of HIV. Its biological activity has been extensively studied, revealing significant efficacy in both pre-exposure prophylaxis (PrEP) and treatment regimens. This article provides a comprehensive overview of this compound's pharmacological properties, clinical efficacy, and safety profile, supported by data tables and case studies.
This compound exhibits a potent antiviral activity against HIV-1, with an effective concentration (EC50) of approximately 0.25 nM for wild-type HIV-1 clade B, indicating its high potency . The pharmacokinetics of this compound demonstrate rapid absorption following oral administration, with over 99.8% plasma protein binding. It undergoes glucuronidation primarily via UGT1A1 and has a terminal elimination half-life of approximately 40 hours .
Table 1: Pharmacokinetic Profile of this compound
| Parameter | Value |
|---|---|
| EC50 (HIV-1 clade B) | 0.25 nM |
| Plasma Protein Binding | >99.8% |
| Terminal Half-Life | ~40 hours |
| Primary Metabolism | UGT1A1 |
Efficacy in Clinical Trials
This compound has been evaluated in several pivotal clinical trials, demonstrating superior efficacy compared to traditional oral PrEP regimens. Notably, the HPTN 083 trial revealed that injectable this compound reduced the risk of HIV acquisition by 66% compared to daily oral tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC) among men who have sex with men and transgender women .
Table 2: Efficacy of this compound in Clinical Trials
| Study | Population | This compound Group Incidence Rate | TDF/FTC Group Incidence Rate | Hazard Ratio (95% CI) |
|---|---|---|---|---|
| HPTN 083 | MSM and Transgender Women | 0.41 per 100 person-years | 1.22 per 100 person-years | 0.34 (0.18–0.62) |
| HPTN 084 | Cisgender Women in Sub-Saharan Africa | 0.21% | 1.79% | 0.11 (0.04–0.32) |
The results from these trials indicate that this compound is significantly more effective than oral PrEP options, particularly in populations with high HIV incidence.
Safety Profile
The safety profile of this compound has been closely monitored in clinical studies. The incidence of adverse events was similar between the this compound and TDF/FTC groups, with no new safety concerns identified during follow-up . However, there were instances of breakthrough infections associated with delayed dosing or missed injections, which raises concerns about adherence and potential resistance development.
Case Studies
Several case studies have highlighted the real-world implications of this compound use in various populations:
- Case Study 1 : In a cohort study involving transgender women, this compound demonstrated high adherence rates and a low incidence of HIV infections despite some participants experiencing treatment interruptions .
- Case Study 2 : A longitudinal study among cisgender women in Eastern Africa reported an incidence rate of only four HIV infections in the this compound group over the study period, showcasing its effectiveness even in high-risk settings .
Q & A
Basic Research Questions
Q. What are the key pharmacokinetic parameters to consider when designing a study on Cabotegravir in diverse populations?
- Methodological Answer : Pharmacokinetic studies should assess parameters such as half-life (), maximum concentration (), and area under the curve (AUC) using validated bioanalytical methods like LC-MS/MS. Population pharmacokinetic modeling (e.g., nonlinear mixed-effects models) can account for covariates like age, weight, and hepatic/renal function. Ensure inclusion of underrepresented subgroups (e.g., pregnant individuals, pediatric populations) to address health equity gaps .
Q. How can researchers optimize in vitro assays to evaluate this compound’s resistance profile?
- Methodological Answer : Use site-directed mutagenesis to introduce known HIV integrase mutations (e.g., Q148H/G140S) into viral strains, followed by phenotypic susceptibility assays. Measure the fold change in EC values compared to wild-type strains. Pair this with structural modeling (e.g., cryo-EM) to correlate mutations with drug-binding affinity changes .
Q. What statistical methods are appropriate for analyzing this compound’s efficacy in prevention versus treatment trials?
- Methodological Answer : For prevention trials (e.g., HPTN 083), use time-to-event analysis (Cox proportional hazards models) with HIV seroconversion as the endpoint. In treatment trials, employ virologic suppression rates (HIV RNA <50 copies/mL) with logistic regression, adjusting for adherence metrics. Address confounding using propensity score matching or inverse probability weighting .
Advanced Research Questions
Q. How can researchers resolve contradictory data on this compound’s efficacy in women versus men?
- Methodological Answer : Conduct stratified subgroup analyses by sex, controlling for variables like body mass index (BMI) and hormonal fluctuations. Use pharmacokinetic-pharmacodynamic (PK/PD) modeling to assess sex-based differences in drug distribution. Design prospective studies with balanced enrollment and longitudinal sampling to capture hormonal cycle effects .
Q. What experimental designs address this compound’s long-acting formulation challenges in real-world adherence studies?
- Methodological Answer : Implement mixed-methods approaches:
- Quantitative : Track injection intervals via electronic medical records and correlate with viral load suppression.
- Qualitative : Conduct semi-structured interviews to identify barriers to clinic attendance (e.g., stigma, accessibility). Use triangulation to reconcile discrepancies between self-reported adherence and pharmacokinetic tail data .
Q. How can in vitro findings on this compound’s drug-drug interactions (DDIs) be translated to clinical practice?
- Methodological Answer : Perform CYP3A4/5 and UGT1A1 enzyme inhibition/induction assays in vitro. Validate results using physiologically based pharmacokinetic (PBPK) modeling (e.g., Simcyp®). Design clinical DDI trials with probe substrates (e.g., midazolam for CYP3A4) in healthy volunteers, followed by targeted monitoring in vulnerable populations (e.g., TB/HIV co-infected patients on rifampicin) .
Q. What strategies mitigate matrix effects when quantifying this compound in heterogeneous biological samples?
- Methodological Answer : Use stable isotope-labeled internal standards (e.g., this compound-d) to normalize matrix effects in LC-MS/MS. Validate methods across matrices (plasma, CSF, tissue homogenates) via spike-and-recovery experiments. For lipid-rich matrices, employ supported liquid extraction (SLE) to reduce phospholipid interference .
Q. How do researchers design studies to explore this compound’s potential in pre-exposure prophylaxis (PrEP) for non-HIV infections?
- Methodological Answer : Repurpose in silico docking studies to predict this compound’s activity against other viral integrases (e.g., HTLV-1). Develop murine models for dose-ranging studies and compare with existing PrEP agents (e.g., tenofovir). Use adaptive trial designs to expedite phase II/III testing in high-incidence populations .
Q. Data Analysis & Interpretation
Q. How should researchers handle missing data in long-term this compound safety trials?
- Methodological Answer : Apply multiple imputation (MI) for missing adverse event data, assuming missing-at-random (MAR) mechanisms. Perform sensitivity analyses (e.g., pattern-mixture models) to assess robustness. For neuropsychiatric outcomes, integrate patient-reported outcomes (PROs) with clinician assessments to reduce bias .
Q. What meta-analysis frameworks are suitable for synthesizing this compound’s global trial data?
- Methodological Answer : Follow PRISMA guidelines to pool data from trials like HPTN 083/084 and FLAIR. Use random-effects models to account for heterogeneity in dosing regimens and populations. Assess publication bias via funnel plots and Egger’s test. Stratify by region and key demographics to identify equity gaps .
Q. Tables for Key Parameters
Properties
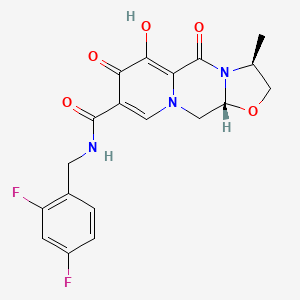
IUPAC Name |
(3R,6S)-N-[(2,4-difluorophenyl)methyl]-10-hydroxy-6-methyl-8,11-dioxo-4-oxa-1,7-diazatricyclo[7.4.0.03,7]trideca-9,12-diene-12-carboxamide | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C19H17F2N3O5/c1-9-8-29-14-7-23-6-12(16(25)17(26)15(23)19(28)24(9)14)18(27)22-5-10-2-3-11(20)4-13(10)21/h2-4,6,9,14,26H,5,7-8H2,1H3,(H,22,27)/t9-,14+/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
WCWSTNLSLKSJPK-LKFCYVNXSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC1COC2N1C(=O)C3=C(C(=O)C(=CN3C2)C(=O)NCC4=C(C=C(C=C4)F)F)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C[C@H]1CO[C@H]2N1C(=O)C3=C(C(=O)C(=CN3C2)C(=O)NCC4=C(C=C(C=C4)F)F)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C19H17F2N3O5 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID50146982 | |
| Record name | GSK-1265744 | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID50146982 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
405.4 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
Cabotegravir binds to the active site of HIV integrase, preventing strand transfer of the viral genome into the host genome, and preventing replication of the virus. | |
| Record name | Cabotegravir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB11751 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
1051375-10-0 | |
| Record name | Cabotegravir | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=1051375-10-0 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Cabotegravir [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=1051375100 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Cabotegravir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB11751 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | GSK-1265744 | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID50146982 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | (3S, 11AR)-N-[(2,4-DIFLUOROPHENYL) METHYL] - 6- HYDROXY-3-METHYL-5,7-DIOXO-2,3,5,7,11,11A- HEXADROOXAZOLO[3,2-A] PYRIDO[1,2-D]PYRAZINE-8-CARBOXAMIDE | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CABOTEGRAVIR | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/HMH0132Z1Q | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.















