瑞德西韦
描述
科学研究应用
Efficacy in Clinical Trials
Numerous clinical trials have evaluated the efficacy of Remdesivir in treating COVID-19. Key findings from several studies are summarized below:
Safety Profile
The safety profile of Remdesivir has been evaluated across multiple studies. Common adverse effects include hepatic impairment; however, the incidence of adverse events was similar between treated and control groups . Ongoing studies continue to monitor long-term safety and efficacy.
Case Study: Efficacy in Severe COVID-19
A systematic review involving over 40 clinical trials indicated that Remdesivir might significantly shorten recovery time among hospitalized adults with severe COVID-19. Observational studies reported reduced mortality rates associated with Remdesivir treatment compared to standard care .
Observational Study: Impact on Mortality Rates
A large-scale observational study involving over 121,000 patients found that Remdesivir-treated individuals had a lower mortality rate within 14 and 28 days compared to those not receiving the drug. The adjusted hazard ratios indicated a statistically significant reduction in mortality associated with Remdesivir use .
Regulatory Approvals and Recommendations
Remdesivir received Emergency Use Authorization from the U.S. Food and Drug Administration for treating COVID-19 in hospitalized patients. The National Institute of Allergy and Infectious Diseases has also endorsed its use based on clinical trial data demonstrating its benefits .
作用机制
GS-5734 通过抑制 RNA 依赖的 RNA 聚合酶 (RdRp) 酶发挥抗病毒作用,该酶对于病毒复制至关重要。进入宿主细胞后,GS-5734 被代谢为其活性三磷酸形式,该形式与天然核苷酸竞争,并被掺入病毒 RNA 中。 这种掺入会导致 RNA 合成过早终止,从而有效地抑制病毒复制 .
生化分析
Biochemical Properties
Remdesivir interacts with the viral RNA-dependent RNA polymerase, an enzyme crucial for viral replication . It is metabolized by the host cell into its active form, a triphosphate (TP) metabolite . This metabolite inhibits viral replication by acting as a chain terminator during the synthesis of viral RNA .
Cellular Effects
Remdesivir has been shown to inhibit the replication of various coronaviruses in human airway epithelial cells . It reduces viral load and improves clinical signs of disease as well as respiratory function .
Molecular Mechanism
The active triphosphate metabolite of Remdesivir incorporates into the growing RNA chain during viral replication. This causes premature termination of the RNA synthesis, thereby inhibiting the replication of the virus . It’s worth noting that Remdesivir can exert its effects even in the presence of the viral proofreading exoribonuclease, an enzyme that often complicates the development of antiviral nucleosides .
Temporal Effects in Laboratory Settings
In laboratory settings, Remdesivir has shown to reduce viral RNA levels in a dose-dependent manner that parallels impairment of viral titer . While it is highly active against wild-type viruses, it has been found to be even more active against viruses lacking the proofreading activity of the exoribonuclease .
Dosage Effects in Animal Models
In animal models, both prophylactic and early therapeutic administration of Remdesivir have significantly reduced lung viral load and improved clinical signs of disease . A study on male mice suggested that a high dosage of Remdesivir may induce testicular toxicity and result in deterioration of sperm parameters .
Metabolic Pathways
Remdesivir is a prodrug that requires metabolism by the host cell to form the pharmacologically active triphosphate metabolite
Transport and Distribution
As a prodrug, it is designed to deliver the nucleoside monophosphate into the cell, thereby circumventing the rate-limiting first phosphorylation step and allowing for efficient intracellular delivery .
准备方法
合成路线和反应条件
GS-5734 的合成涉及多个步骤,从商业上可获得的起始材料开始。关键步骤包括核苷类似物的形成及其随后的磷酸化以产生活性三磷酸形式。 合成路线通常涉及保护和去保护步骤、亲核取代反应以及在受控条件下的磷酸化反应 .
工业生产方法
GS-5734 的工业生产遵循类似的合成路线,但针对大规模生产进行了优化。 这涉及使用高产反应、高效纯化方法以及严格的质量控制措施,以确保最终产品的稳定性和纯度 .
化学反应分析
反应类型
GS-5734 经历了几种类型的化学反应,包括:
氧化: GS-5734 可以氧化形成各种代谢物。
还原: 还原反应可以改变核苷类似物的结构。
常见试剂和条件
在 GS-5734 的合成和反应中使用的常见试剂包括:
磷酸化试剂: 用于磷酸化步骤。
保护基团: 用于在合成过程中保护官能团。
形成的主要产物
相似化合物的比较
类似化合物
GS-441524: GS-5734 的母体核苷类似物,具有类似的抗病毒活性。
法匹拉韦: 另一种具有广谱抗病毒活性的核苷类似物。
利巴韦林: 一种用于治疗各种病毒感染的核苷类似物.
GS-5734 的独特性
GS-5734 的独特之处在于其广谱抗病毒活性以及抑制多种 RNA 病毒的能力,包括冠状病毒和丝状病毒。 它对 SARS-CoV-2 的有效性使其成为对抗 COVID-19 的重要组成部分 .
生物活性
Remdesivir, a nucleotide analog prodrug, has garnered significant attention as a therapeutic agent against viral infections, particularly COVID-19. This article delves into the biological activity of remdesivir, focusing on its mechanism of action, pharmacokinetics, clinical efficacy, and ongoing research.
Remdesivir (GS-5734) is designed to inhibit viral RNA-dependent RNA polymerase (RdRp), crucial for the replication of RNA viruses. Upon entering host cells, remdesivir is metabolized into its active form, GS-443902, which mimics adenosine triphosphate (ATP). This active form competes with ATP for incorporation into the viral RNA strand during replication. The incorporation of GS-443902 leads to premature termination of RNA synthesis, thereby limiting viral replication.
Key Mechanisms:
- Nucleotide Analog: Resembles ATP and integrates into viral RNA.
- Chain Termination: Causes premature termination of RNA synthesis.
- Broad Spectrum Activity: Effective against various coronaviruses (SARS-CoV, MERS-CoV) and other RNA viruses like Ebola .
Pharmacokinetics
The pharmacokinetics of remdesivir have been studied primarily through intravenous administration. A randomized controlled trial indicated that remdesivir exhibits linear pharmacokinetics across various doses (3 to 225 mg). Notably, it is a substrate for organic anion transporting polypeptides (OATP1B1 and OATP1B3), influencing its hepatic uptake and elimination .
Table 1: Pharmacokinetic Profile of Remdesivir
| Parameter | Value |
|---|---|
| Bioavailability | Not applicable (IV only) |
| Half-life | Approximately 1 hour |
| Volume of distribution | 1 L/kg |
| Clearance | 0.5 L/h/kg |
Clinical Efficacy
Several clinical trials have evaluated the efficacy of remdesivir in treating COVID-19. The most notable studies include:
- ACTT-1 Trial: A randomized trial demonstrating that patients receiving remdesivir had a shorter time to recovery compared to those receiving standard care.
- SIMPLE Trials: These trials assessed the efficacy of 5-day versus 10-day treatment regimens in hospitalized patients with severe COVID-19. Results indicated that both regimens provided similar clinical improvements .
Table 2: Summary of Key Clinical Trials
Case Studies and Observational Data
Observational studies have also provided insights into the efficacy and safety profile of remdesivir. For instance, a study involving hospitalized patients showed that those treated with a 5-day course had statistically significant improvements in clinical status at Day 11 compared to those receiving standard care .
Case Study Example:
A cohort study analyzed outcomes in critically ill patients treated with remdesivir. It found that early administration correlated with improved survival rates and reduced need for mechanical ventilation.
Ongoing Research and Future Directions
Research continues to explore the full potential of remdesivir beyond COVID-19. Current studies are investigating:
- Combination therapies with other antiviral agents.
- Efficacy in pediatric populations.
- Alternative formulations to enhance delivery methods.
属性
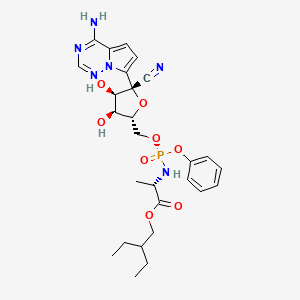
IUPAC Name |
2-ethylbutyl (2S)-2-[[[(2R,3S,4R,5R)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxyoxolan-2-yl]methoxy-phenoxyphosphoryl]amino]propanoate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C27H35N6O8P/c1-4-18(5-2)13-38-26(36)17(3)32-42(37,41-19-9-7-6-8-10-19)39-14-21-23(34)24(35)27(15-28,40-21)22-12-11-20-25(29)30-16-31-33(20)22/h6-12,16-18,21,23-24,34-35H,4-5,13-14H2,1-3H3,(H,32,37)(H2,29,30,31)/t17-,21+,23+,24+,27-,42-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
RWWYLEGWBNMMLJ-YSOARWBDSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCC(CC)COC(=O)C(C)NP(=O)(OCC1C(C(C(O1)(C#N)C2=CC=C3N2N=CN=C3N)O)O)OC4=CC=CC=C4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CCC(CC)COC(=O)[C@H](C)N[P@](=O)(OC[C@@H]1[C@H]([C@H]([C@](O1)(C#N)C2=CC=C3N2N=CN=C3N)O)O)OC4=CC=CC=C4 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C27H35N6O8P | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID701022537 | |
| Record name | Remdesivir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID701022537 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
602.6 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
COVID-19 is caused by the positive-sense RNA virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Replication of the viral genome is a key step in the infectious cycle of RNA viruses, including those of the _Filoviridae_, _Paramyxoviridae_, _Pneumoviridae_, and _Coronaviridae_ families, and is carried out by viral RNA-dependent RNA polymerase (RdRp) enzymes or enzyme complexes. For both SARS-CoV and SARS-CoV-2, the RdRp comprises nsp7, nsp8, and nsp12 subunits under physiological conditions, although functional RdRp complexes can be reassembled _in vitro_ that incorporate only the nsp8 and nsp12 subunits, similar to the Middle East respiratory syndrome coronavirus (MERS-CoV). Remdesivir is a phosphoramidite prodrug of a 1'-cyano-substituted adenosine nucleotide analogue that competes with ATP for incorporation into newly synthesized viral RNA by the corresponding RdRp complex. Remdesivir enters cells before being cleaved to its monophosphate form through the action of either carboxylesterase 1 or cathepsin A; it is subsequently phosphorylated by undescribed kinases to yield its active triphosphate form remdesivir triphosphate (RDV-TP or GS-443902). RDV-TP is efficiently incorporated by the SARS-CoV-2 RdRp complex, with a 3.65-fold selectivity for RDV-TP over endogenous ATP. Unlike some nucleoside analogues, remdesivir provides a free 3'-hydroxyl group that allows for continued chain elongation. However, modelling and _in vitro_ experiments suggest that at _i_ + 4 (corresponding to the position for the incorporation of the fourth nucleotide following RDV-TP incorporation), the 1'-cyano group of remdesivir sterically clashes with Ser-861 of the RdRp, preventing further enzyme translocation and terminating replication at position _i_ + 3. This mechanism was essentially identical between SARS-CoV, SARS-CoV-2, and MERS-CoV, and genomic comparisons reveal that Ser-861 is conserved across alpha-, beta-, and deltacoronaviruses, suggesting remdesivir may possess broad antiviral activity. Considerations for the use of nucleotide analogues like remdesivir include the possible accumulation of resistance mutations. Excision of analogues through the 3'-5' exonuclease (ExoN) activity of replication complexes, mediated in SARS-CoV by the nsp14 subunit, is of possible concern. Murine hepatitis viruses (MHVs) engineered to lack ExoN activity are approximately 4-fold more susceptible to remdesivir, supporting the proposed mechanism of action. However, the relatively mild benefit of ExoN activity to remdesivir resistance is proposed to involve its delayed chain termination mechanism, whereby additional endogenous nucleotides are incorporated following RDV-TP. In addition, serial passage of MHV in increasing concentrations of the remdesivir parent molecule [GS-441524] led to the development of resistance mutations F476L and V553L, which maintain activity when transferred to SARS-CoV. However, these mutant viruses are less fit than wild-type in both competition assays and _in vivo_ in the absence of selective pressure. To date, no clinical data on SARS-CoV-2 resistance to remdesivir have been described. | |
| Record name | Remdesivir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB14761 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
1809249-37-3 | |
| Record name | L-Alanine, N-[(S)-hydroxyphenoxyphosphinyl]-, 2-ethylbutyl ester, 6-ester with 2-C-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-2,5-anhydro-D-altrononitrile | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=1809249-37-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Remdesivir [USAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=1809249373 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Remdesivir | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB14761 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Remdesivir | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID701022537 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | 2-ethylbutyl (2S)-2-[[[(2R,3S,4R,5R)-5-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-5-cyano-3,4-dihydroxyoxolan-2-yl]methoxy-phenoxyphosphoryl]amino]propanoate | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | REMDESIVIR | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/3QKI37EEHE | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。















