Miltefosin
Übersicht
Beschreibung
Miltefosin ist ein Breitband-Antibiotikum und Anti-Leishmaniose-Mittel. Ursprünglich in den 1980er Jahren als Antikrebsmittel entwickelt, wird es heute hauptsächlich zur Behandlung von Leishmaniose eingesetzt, einer Krankheit, die durch Parasiten der Leishmania-Art verursacht wird. This compound ist das erste und einzige orale Medikament, das für die Behandlung von viszeralen, kutanen und mukosalen Formen der Leishmaniose zugelassen ist . Es wird auch off-label zur Behandlung von Infektionen verwendet, die durch frei lebende Amöben verursacht werden .
Wissenschaftliche Forschungsanwendungen
Miltefosine is an alkylphosphocholine drug with activity against some pathogenic bacteria and fungi, various parasite species, and cancer cells . It is the first oral drug and remains the only one available for treating visceral leishmaniasis (VL) and cutaneous leishmaniasis (CL) . Miltefosine was initially developed as an anticancer agent in the 1990s and was registered in India in 2002 for treating visceral leishmaniasis .
Pharmacology of Miltefosine
Miltefosine has demonstrated activity against neoplastic cells and Leishmania parasites, primarily due to its effects on apoptosis and disturbance of lipid-dependent cell signalling pathways . While several potential antileishmanial mechanisms of action have been proposed, none has been definitively identified . Miltefosine inhibits cytochrome-c oxidase within the mitochondria, which leads to mitochondrial dysfunction and apoptosis-like cell death . The antineoplastic mechanisms of action are related to antileishmanial targets, including inhibition of phosphatidylcholine biosynthesis and inhibition of Akt (protein kinase B), a crucial protein within the PI3K/Akt/mTOR intracellular signalling pathway that regulates the cell cycle .
Indications
Miltefosine is indicated for treating mucosal leishmaniasis (caused by Leishmania braziliensis), cutaneous leishmaniasis (caused by L. braziliensis, L. guyanensis, and L. panamensis), and visceral leishmaniasis (caused by L. donovani) . L. donovani is the most susceptible to miltefosine, while L. major is the least susceptible among Leishmania species . Miltefosine is also used off-label to treat free-living amoebae (FLA) infections . Animal studies suggest it may be effective against metronidazole-resistant strains of Trichonomas vaginalis, Trypanosome cruzi (responsible for Chagas' disease), and may have broad-spectrum antifungal activity .
Clinical Trials and Efficacy
Miltefosine has demonstrated high cure rates for visceral leishmaniasis in clinical trials performed in India, with 94%–97% cure rates using regimens of 2.5 mg/kg/day for 28 days . Clinical trials in India involving children under 12 years of age have shown 83%–94% cure rates . A trial in Bangladesh, including adults and children, achieved 85% cure rates .
Adverse Effects
The standard 28-day miltefosine monotherapy regimen is well-tolerated, though it has some side effects, such as mild gastrointestinal issues . Common adverse events include nausea/vomiting (97%) and lack of appetite (54%) . Clinical management or dose reduction was required in a third of cases . Most laboratory abnormalities, including elevated creatinine and aminotransferases, were mild and normalized after treatment .
Leishmaniasis Treatment
Miltefosine is the only recognized oral agent with the potential to treat leishmaniasis . It has demonstrated very good cure rates for visceral leishmaniasis in India, Nepal, and Bangladesh . However, there have been reports of high rates of clinical failures recently . In East Africa, it has moderate efficacy for VL, while data from Mediterranean countries and Latin America are scarce .
Good evidence of efficacy has been documented in Old World cutaneous leishmaniasis . Different cure rates among New World CL have been obtained depending on the geographical areas and species involved . Appropriate regimens for New World mucocutaneous leishmaniasis need to be established, although longer treatment duration seems to confer better results .
Case Studies
- Cutaneous Leishmaniasis: A study showed that miltefosine may be an effective and safe oral medication for treating Old World cutaneous leishmaniasis caused by L. major .
- Primary Amoebic Meningoencephalitis (PAM): Miltefosine has been used in cases of PAM, with some reports of successful outcomes . In one report, a man with no comorbidities presented with fever, headache, and generalized weakness and was diagnosed with PAM within 24 hours. The CDC guideline was implemented on the first day. On day three, he developed diabetes insipidus (DI) and died four days later with cardiac arrest .
- Vitiligo: Autologous non-cultured basal-enriched epidermal cell suspension transplantation with some modifications is an effective, simple, and safe method for treating stable vitiligo .
Tables
| Leishmania Species | Region/Country | Cure Rate |
|---|---|---|
| L. panamensis | Costa Rica | 77% |
| L. braziliensis | Peru, Belize | 77% |
| L. mexicana | Ecuador | 77% |
| L. infantum | El Salvador, Spain, Italy | 77% |
| L. major | Morocco | 77% |
| L. tropica | Afghanistan, Pakistan | 77% |
| L. aethiopica | Ethiopia | 77% |
| Unspecified | Honduras, Suriname | 77% |
Resistance
Wirkmechanismus
Miltefosine is an alkylphosphocholine drug with demonstrated activity against various parasite species and cancer cells . It is the only oral drug approved for the treatment of Leishmaniasis and American Trypanosomiasis (Chagas disease) .
Target of Action
Miltefosine’s primary targets are the Leishmania parasites and neoplastic cells . It has a broad-spectrum anti-parasitic effect, primarily disrupting the intracellular Ca2+ homeostasis of the parasites while sparing the human hosts .
Mode of Action
Miltefosine interacts with its targets primarily through two mechanisms :
- Apoptosis : Miltefosine induces apoptosis-like cell death in its targets .
- Disturbance of lipid-dependent cell signaling pathways : Miltefosine interferes with the functioning of several enzymes involved in phospholipid metabolism .
Biochemical Pathways
Miltefosine affects the unique giant mitochondria and the acidocalcisomes of parasites, both of which are involved in Ca2+ regulation . It inhibits phosphatidylcholine biosynthesis in mammalian cells, primarily via the Kennedy CDP–Choline pathway, by blocking phosphocholine citidyltransferase . In Trypanosoma cruzi, miltefosine inhibits the Greenberg (transmethylation) pathway by acting on phosphatidylethanolamine N methyl-transferase .
Pharmacokinetics
Miltefosine is characterized by slow absorption and elimination, leading to long initial (approximately 7 days) and terminal (approximately 30 days) half-lives . It is not a substrate of cytochrome P450 metabolic enzymes and only 0.2% of the administered dose is eliminated in the urine at day 23 of a 28-day treatment regimen . The absorption of miltefosine is concentration-dependent, with passive paracellular diffusion applicable to the concentration below 20.4 μg/mL .
Result of Action
Miltefosine exhibits broad-spectrum anti-parasitic effects primarily by disrupting the intracellular Ca2+ homeostasis of the parasites . It also positively affects the host’s immune system . The drug interferes with biosynthesis of phospholipids and metabolism of alkyl-lipids . It is known to cause cell shrinkage, nuclear DNA condensation, and DNA fragmentation resulting in apoptosis-like cell death in L. donovani .
Action Environment
It is known that the drug’s therapeutic effect extends beyond its impact on the parasite to also positively affect the host’s immune system . These findings suggest a complex interplay between drug susceptibility, neutrophil activation, and Leishmania survival .
Biochemische Analyse
Biochemical Properties
Miltefosine plays a significant role in biochemical reactions. It interacts with various enzymes, proteins, and other biomolecules. The compound is metabolized mainly by phospholipase D, releasing choline, choline-containing metabolites, and hexadecanol . These metabolites are all endogenous and are likely used for the biosynthesis of acetylcholine, cell membranes, and long-chain fatty acids .
Cellular Effects
Miltefosine has profound effects on various types of cells and cellular processes. It influences cell function, including impacts on cell signaling pathways, gene expression, and cellular metabolism . It has been found to affect the unique giant mitochondria and the acidocalcisomes of parasites, both of which are involved in Ca 2+ regulation .
Molecular Mechanism
The molecular mechanism of action of Miltefosine involves its binding interactions with biomolecules, enzyme inhibition or activation, and changes in gene expression . In addition to its inhibitory effects on phosphatidylcholine synthesis and cytochrome c oxidase, miltefosine has been found to affect the unique giant mitochondria and the acidocalcisomes of parasites .
Dosage Effects in Animal Models
The effects of Miltefosine vary with different dosages in animal models . This includes any threshold effects observed in these studies, as well as any toxic or adverse effects at high doses .
Metabolic Pathways
Miltefosine is involved in several metabolic pathways. It interacts with enzymes or cofactors, and it can also affect metabolic flux or metabolite levels .
Transport and Distribution
Miltefosine is transported and distributed within cells and tissues . This includes any transporters or binding proteins that it interacts with, as well as any effects on its localization or accumulation .
Subcellular Localization
This could include any targeting signals or post-translational modifications that direct it to specific compartments or organelles .
Vorbereitungsmethoden
Synthesewege und Reaktionsbedingungen
Miltefosin kann durch einen mehrstufigen Prozess synthetisiert werden. Eine gängige Methode beinhaltet die Reaktion von Cetylalkohol mit Triethylamin und Trichlorphosphinoxid in Tetrahydrofuran. Anschließend wird Dimethylethanolamin und Triethylamin zugegeben, um Cetylphosphoramid zu erhalten .
Industrielle Produktionsmethoden
Die industrielle Produktion von this compound beinhaltet typischerweise die großtechnische Synthese unter Verwendung ähnlicher chemischer Reaktionen wie oben beschrieben. Der Prozess ist auf hohe Ausbeute und Reinheit optimiert, um sicherzustellen, dass das Endprodukt pharmazeutische Standards erfüllt .
Analyse Chemischer Reaktionen
Arten von Reaktionen
Miltefosin unterliegt verschiedenen chemischen Reaktionen, darunter Oxidation, Reduktion und Substitution. Diese Reaktionen sind für seinen Stoffwechsel und seine biologische Aktivität unerlässlich .
Häufige Reagenzien und Bedingungen
Häufige Reagenzien, die bei den Reaktionen mit this compound verwendet werden, sind Oxidationsmittel, Reduktionsmittel und verschiedene Lösungsmittel. Die Bedingungen für diese Reaktionen werden sorgfältig kontrolliert, um die gewünschten Ergebnisse zu erzielen .
Hauptprodukte, die gebildet werden
Die Hauptprodukte, die aus den Reaktionen von this compound gebildet werden, hängen von den verwendeten Reagenzien und Bedingungen ab. Beispielsweise können Oxidationsreaktionen verschiedene Metaboliten produzieren, die zu seinen therapeutischen Wirkungen beitragen .
Wissenschaftliche Forschungsanwendungen
This compound hat eine breite Palette wissenschaftlicher Forschungsanwendungen:
Medizin: this compound wird zur Behandlung von Leishmaniose und Infektionen mit frei lebenden Amöben eingesetzt. .
Wirkmechanismus
This compound übt seine Wirkungen aus, indem es die intrazellulären Membranen von Parasiten stört, was zum Zelltod führt. Es hemmt die Synthese von Phosphatidylcholin und beeinflusst die intrazelluläre Calciumhomöostase, die für das Überleben von Parasiten entscheidend ist . Das Medikament zielt auch auf Cytochrom-c-Oxidase und andere essentielle Enzyme ab, was zu seiner antiparasitären Aktivität beiträgt .
Vergleich Mit ähnlichen Verbindungen
Miltefosin gehört zur Klasse der Alkylphosphorcholinkomponenten. Ähnliche Verbindungen umfassen Hexadecylphosphorcholin und andere Alkylglycerophosphocholine. Diese Verbindungen teilen strukturelle Ähnlichkeiten, unterscheiden sich jedoch in ihrer Alkylkettenlänge und ihrer Rückgratstruktur . Die einzigartige Kombination von Eigenschaften von this compound macht es besonders wirksam gegen Leishmaniose und andere parasitäre Infektionen .
Liste ähnlicher Verbindungen
- Hexadecylphosphorcholin
- Alkylglycerophosphocholine
- Etherlipide
This compound zeichnet sich durch seine orale Bioverfügbarkeit und seine Breitbandaktivität aus, was es zu einem wertvollen Werkzeug im Kampf gegen parasitäre Krankheiten macht .
Biologische Aktivität
Miltefosine is a phospholipid analog primarily used in the treatment of leishmaniasis, a parasitic disease caused by protozoa of the genus Leishmania. This compound has gained attention due to its unique mechanism of action, efficacy, and the emergence of resistance in some cases. The following sections will detail its biological activity, mechanisms, clinical efficacy, and implications for future treatments.
Miltefosine's biological activity is multifaceted, impacting both the parasite and the host's immune system. Key aspects include:
- Disruption of Lipid Metabolism : Miltefosine inhibits phosphatidylcholine synthesis, crucial for maintaining cellular membrane integrity in parasites. This disruption leads to cell death in Leishmania species .
- Calcium Homeostasis : The drug significantly affects calcium ion regulation within the parasites. It activates specific calcium channels that increase intracellular calcium levels, disrupting mitochondrial function and leading to apoptosis .
- Immune Modulation : Miltefosine has been shown to upregulate immune markers such as CD16 and CD86 on monocytes while downregulating CD14. This modulation may enhance the host's immune response against Leishmania infections .
Clinical Efficacy
Miltefosine is the first oral medication approved for treating leishmaniasis. Its efficacy has been documented across various studies:
- Visceral Leishmaniasis (VL) : Initial studies reported cure rates of approximately 94% during early trials. However, recent data indicate a decline in efficacy, with cure rates dropping to around 90% over a decade of use .
- Post Kala-Azar Dermal Leishmaniasis (PKDL) : In a study involving 86 patients treated with miltefosine, 85% achieved clinical cure after 18 months, although relapse rates were notably higher in patients with greater pre-treatment parasite loads .
- Cutaneous Leishmaniasis : A case series demonstrated an overall cure rate of 77% among various Leishmania species treated with miltefosine. Adverse effects included nausea and vomiting, which were common but manageable .
Case Study 1: Efficacy in PKDL
A five-year study on PKDL patients showed that miltefosine treatment resulted in an initial cure rate of 78%. However, follow-up revealed a significant relapse rate linked to higher parasite loads at baseline. The study underscores the importance of monitoring parasite susceptibility to miltefosine over time .
Case Study 2: Cutaneous Leishmaniasis
In a cohort treated for cutaneous leishmaniasis, miltefosine resulted in an 80% complete response rate. The treatment was well-tolerated overall, although some patients experienced significant side effects requiring dose adjustments .
Resistance Concerns
The emergence of resistance to miltefosine poses a significant challenge. Studies have shown that parasites isolated from relapsed patients exhibited more than two-fold tolerance to miltefosine compared to pre-treatment isolates. This highlights the necessity for combination therapies to mitigate resistance risks and improve treatment outcomes .
Summary Table of Efficacy Data
| Condition | Initial Cure Rate | Relapse Rate | Notable Side Effects |
|---|---|---|---|
| Visceral Leishmaniasis | ~94% | ~6.8% | Nausea, vomiting |
| Post Kala-Azar Dermal Leishmaniasis | ~85% | Higher with increased parasite load | Mild gastrointestinal issues |
| Cutaneous Leishmaniasis | ~77% | Not specified | Nausea (97%), lack of appetite (54%) |
Eigenschaften
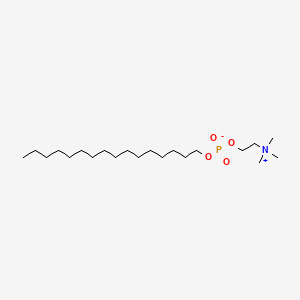
IUPAC Name |
hexadecyl 2-(trimethylazaniumyl)ethyl phosphate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C21H46NO4P/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-20-25-27(23,24)26-21-19-22(2,3)4/h5-21H2,1-4H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
PQLXHQMOHUQAKB-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCCCCCCCCCCCCCCCOP(=O)([O-])OCC[N+](C)(C)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C21H46NO4P | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID7045942 | |
| Record name | Miltefosine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7045942 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
407.6 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
Miltefosine has demonstrated activity against Leishmania parasites and neoplastic cells primarily due to its effects on apoptosis and disturbance of lipid-dependent cell signalling pathways. Several potential antileishmanial mechanisms of action have been proposed, however no mechanism has been identified definitely. Within the mitochondria, miltefosine inhibits cytochrome-c oxidase leading to mitochondrial dysfunction and apoptosis-like cell death. Antineoplastic mechanisms of action are related to antileishmanial targets and include inhibition of phosphatidylcholine biosynthesis and inhibition of Akt (also known as protein kinase B), which is a crucial protein within the PI3K/Akt/mTOR intracellular signalling pathway involved in regulating the cell cycle. Animal studies also suggest it may be effective against Trypanosome cruzi (the organism responsible for Chagas' disease), metronidazole-resistant strains of Trichonomas vaginalis, and it may have broad-spectrum anti-fungal activity. | |
| Record name | Miltefosine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09031 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
58066-85-6 | |
| Record name | Miltefosine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=58066-85-6 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Miltefosine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0058066856 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Miltefosine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09031 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Miltefosine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=758968 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Miltefosine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7045942 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Miltefosine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | MILTEFOSINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/53EY29W7EC | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.















