Miltefosine
Vue d'ensemble
Description
La miltéfosine est un agent antimicrobien et antileishmanien à large spectre. Initialement développé dans les années 1980 comme médicament anticancéreux, il est maintenant principalement utilisé pour traiter la leishmaniose, une maladie causée par des parasites du type Leishmania. La miltéfosine est le premier et le seul médicament oral approuvé pour le traitement des formes viscérale, cutanée et muqueuse de la leishmaniose . Elle est également utilisée en off-label pour traiter les infections causées par des amibes libres .
Mécanisme D'action
Miltefosine is an alkylphosphocholine drug with demonstrated activity against various parasite species and cancer cells . It is the only oral drug approved for the treatment of Leishmaniasis and American Trypanosomiasis (Chagas disease) .
Target of Action
This compound’s primary targets are the Leishmania parasites and neoplastic cells . It has a broad-spectrum anti-parasitic effect, primarily disrupting the intracellular Ca2+ homeostasis of the parasites while sparing the human hosts .
Mode of Action
This compound interacts with its targets primarily through two mechanisms :
- Apoptosis : this compound induces apoptosis-like cell death in its targets .
- Disturbance of lipid-dependent cell signaling pathways : this compound interferes with the functioning of several enzymes involved in phospholipid metabolism .
Biochemical Pathways
This compound affects the unique giant mitochondria and the acidocalcisomes of parasites, both of which are involved in Ca2+ regulation . It inhibits phosphatidylcholine biosynthesis in mammalian cells, primarily via the Kennedy CDP–Choline pathway, by blocking phosphocholine citidyltransferase . In Trypanosoma cruzi, this compound inhibits the Greenberg (transmethylation) pathway by acting on phosphatidylethanolamine N methyl-transferase .
Pharmacokinetics
This compound is characterized by slow absorption and elimination, leading to long initial (approximately 7 days) and terminal (approximately 30 days) half-lives . It is not a substrate of cytochrome P450 metabolic enzymes and only 0.2% of the administered dose is eliminated in the urine at day 23 of a 28-day treatment regimen . The absorption of this compound is concentration-dependent, with passive paracellular diffusion applicable to the concentration below 20.4 μg/mL .
Result of Action
This compound exhibits broad-spectrum anti-parasitic effects primarily by disrupting the intracellular Ca2+ homeostasis of the parasites . It also positively affects the host’s immune system . The drug interferes with biosynthesis of phospholipids and metabolism of alkyl-lipids . It is known to cause cell shrinkage, nuclear DNA condensation, and DNA fragmentation resulting in apoptosis-like cell death in L. donovani .
Action Environment
It is known that the drug’s therapeutic effect extends beyond its impact on the parasite to also positively affect the host’s immune system . These findings suggest a complex interplay between drug susceptibility, neutrophil activation, and Leishmania survival .
Analyse Biochimique
Biochemical Properties
Miltefosine plays a significant role in biochemical reactions. It interacts with various enzymes, proteins, and other biomolecules. The compound is metabolized mainly by phospholipase D, releasing choline, choline-containing metabolites, and hexadecanol . These metabolites are all endogenous and are likely used for the biosynthesis of acetylcholine, cell membranes, and long-chain fatty acids .
Cellular Effects
This compound has profound effects on various types of cells and cellular processes. It influences cell function, including impacts on cell signaling pathways, gene expression, and cellular metabolism . It has been found to affect the unique giant mitochondria and the acidocalcisomes of parasites, both of which are involved in Ca 2+ regulation .
Molecular Mechanism
The molecular mechanism of action of this compound involves its binding interactions with biomolecules, enzyme inhibition or activation, and changes in gene expression . In addition to its inhibitory effects on phosphatidylcholine synthesis and cytochrome c oxidase, this compound has been found to affect the unique giant mitochondria and the acidocalcisomes of parasites .
Dosage Effects in Animal Models
The effects of this compound vary with different dosages in animal models . This includes any threshold effects observed in these studies, as well as any toxic or adverse effects at high doses .
Metabolic Pathways
This compound is involved in several metabolic pathways. It interacts with enzymes or cofactors, and it can also affect metabolic flux or metabolite levels .
Transport and Distribution
This compound is transported and distributed within cells and tissues . This includes any transporters or binding proteins that it interacts with, as well as any effects on its localization or accumulation .
Subcellular Localization
This could include any targeting signals or post-translational modifications that direct it to specific compartments or organelles .
Méthodes De Préparation
Voies de synthèse et conditions de réaction
La miltéfosine peut être synthétisée par un processus en plusieurs étapes. Une méthode courante implique la réaction de l'alcool cétylique avec la triéthylamine et l'oxyde de trichlorure de phosphore dans le tétrahydrofurane. Ceci est suivi de l'addition de diméthyléthanolamine et de triéthylamine pour obtenir le phosphamide de cétyle .
Méthodes de production industrielle
La production industrielle de la miltéfosine implique généralement une synthèse à grande échelle utilisant des réactions chimiques similaires à celles décrites ci-dessus. Le processus est optimisé pour un rendement élevé et une pureté élevée, garantissant que le produit final répond aux normes pharmaceutiques .
Analyse Des Réactions Chimiques
Types de réactions
La miltéfosine subit diverses réactions chimiques, notamment l'oxydation, la réduction et la substitution. Ces réactions sont essentielles pour son métabolisme et son activité biologique .
Réactifs et conditions courants
Les réactifs courants utilisés dans les réactions impliquant la miltéfosine comprennent les oxydants, les réducteurs et divers solvants. Les conditions de ces réactions sont soigneusement contrôlées pour assurer les résultats souhaités .
Principaux produits formés
Les principaux produits formés à partir des réactions de la miltéfosine dépendent des réactifs et des conditions spécifiques utilisés. Par exemple, les réactions d'oxydation peuvent produire différents métabolites qui contribuent à ses effets thérapeutiques .
Applications de la recherche scientifique
La miltéfosine a un large éventail d'applications de recherche scientifique :
Médecine : La miltéfosine est utilisée pour traiter la leishmaniose et les infections par des amibes libres. .
Mécanisme d'action
La miltéfosine exerce ses effets en perturbant les membranes intracellulaires des parasites, ce qui conduit à la mort cellulaire. Elle inhibe la synthèse de la phosphatidylcholine et affecte l'homéostasie du calcium intracellulaire, ce qui est crucial pour la survie des parasites . Le médicament cible également la cytochrome c oxydase et d'autres enzymes essentielles, contribuant ainsi à son activité antiparasitaire .
Applications De Recherche Scientifique
Miltefosine is an alkylphosphocholine drug with activity against some pathogenic bacteria and fungi, various parasite species, and cancer cells . It is the first oral drug and remains the only one available for treating visceral leishmaniasis (VL) and cutaneous leishmaniasis (CL) . this compound was initially developed as an anticancer agent in the 1990s and was registered in India in 2002 for treating visceral leishmaniasis .
Pharmacology of this compound
This compound has demonstrated activity against neoplastic cells and Leishmania parasites, primarily due to its effects on apoptosis and disturbance of lipid-dependent cell signalling pathways . While several potential antileishmanial mechanisms of action have been proposed, none has been definitively identified . this compound inhibits cytochrome-c oxidase within the mitochondria, which leads to mitochondrial dysfunction and apoptosis-like cell death . The antineoplastic mechanisms of action are related to antileishmanial targets, including inhibition of phosphatidylcholine biosynthesis and inhibition of Akt (protein kinase B), a crucial protein within the PI3K/Akt/mTOR intracellular signalling pathway that regulates the cell cycle .
Indications
This compound is indicated for treating mucosal leishmaniasis (caused by Leishmania braziliensis), cutaneous leishmaniasis (caused by L. braziliensis, L. guyanensis, and L. panamensis), and visceral leishmaniasis (caused by L. donovani) . L. donovani is the most susceptible to this compound, while L. major is the least susceptible among Leishmania species . this compound is also used off-label to treat free-living amoebae (FLA) infections . Animal studies suggest it may be effective against metronidazole-resistant strains of Trichonomas vaginalis, Trypanosome cruzi (responsible for Chagas' disease), and may have broad-spectrum antifungal activity .
Clinical Trials and Efficacy
This compound has demonstrated high cure rates for visceral leishmaniasis in clinical trials performed in India, with 94%–97% cure rates using regimens of 2.5 mg/kg/day for 28 days . Clinical trials in India involving children under 12 years of age have shown 83%–94% cure rates . A trial in Bangladesh, including adults and children, achieved 85% cure rates .
Adverse Effects
The standard 28-day this compound monotherapy regimen is well-tolerated, though it has some side effects, such as mild gastrointestinal issues . Common adverse events include nausea/vomiting (97%) and lack of appetite (54%) . Clinical management or dose reduction was required in a third of cases . Most laboratory abnormalities, including elevated creatinine and aminotransferases, were mild and normalized after treatment .
Leishmaniasis Treatment
This compound is the only recognized oral agent with the potential to treat leishmaniasis . It has demonstrated very good cure rates for visceral leishmaniasis in India, Nepal, and Bangladesh . However, there have been reports of high rates of clinical failures recently . In East Africa, it has moderate efficacy for VL, while data from Mediterranean countries and Latin America are scarce .
Good evidence of efficacy has been documented in Old World cutaneous leishmaniasis . Different cure rates among New World CL have been obtained depending on the geographical areas and species involved . Appropriate regimens for New World mucocutaneous leishmaniasis need to be established, although longer treatment duration seems to confer better results .
Case Studies
- Cutaneous Leishmaniasis: A study showed that this compound may be an effective and safe oral medication for treating Old World cutaneous leishmaniasis caused by L. major .
- Primary Amoebic Meningoencephalitis (PAM): this compound has been used in cases of PAM, with some reports of successful outcomes . In one report, a man with no comorbidities presented with fever, headache, and generalized weakness and was diagnosed with PAM within 24 hours. The CDC guideline was implemented on the first day. On day three, he developed diabetes insipidus (DI) and died four days later with cardiac arrest .
- Vitiligo: Autologous non-cultured basal-enriched epidermal cell suspension transplantation with some modifications is an effective, simple, and safe method for treating stable vitiligo .
Tables
| Leishmania Species | Region/Country | Cure Rate |
|---|---|---|
| L. panamensis | Costa Rica | 77% |
| L. braziliensis | Peru, Belize | 77% |
| L. mexicana | Ecuador | 77% |
| L. infantum | El Salvador, Spain, Italy | 77% |
| L. major | Morocco | 77% |
| L. tropica | Afghanistan, Pakistan | 77% |
| L. aethiopica | Ethiopia | 77% |
| Unspecified | Honduras, Suriname | 77% |
Resistance
Comparaison Avec Des Composés Similaires
La miltéfosine appartient à la classe des composés alkylphosphocholine. Des composés similaires comprennent l'hexadécylphosphocholine et d'autres alkylglycérophosphocholines. Ces composés présentent des similitudes structurelles mais diffèrent par la longueur de leur chaîne alkyle et la structure de leur squelette . La combinaison unique de propriétés de la miltéfosine la rend particulièrement efficace contre la leishmaniose et d'autres infections parasitaires .
Liste des composés similaires
- Hexadécylphosphocholine
- Alkylglycérophosphocholines
- Lipides éther
La miltéfosine se distingue par sa biodisponibilité orale et son activité à large spectre, ce qui en fait un outil précieux dans la lutte contre les maladies parasitaires .
Activité Biologique
Miltefosine is a phospholipid analog primarily used in the treatment of leishmaniasis, a parasitic disease caused by protozoa of the genus Leishmania. This compound has gained attention due to its unique mechanism of action, efficacy, and the emergence of resistance in some cases. The following sections will detail its biological activity, mechanisms, clinical efficacy, and implications for future treatments.
This compound's biological activity is multifaceted, impacting both the parasite and the host's immune system. Key aspects include:
- Disruption of Lipid Metabolism : this compound inhibits phosphatidylcholine synthesis, crucial for maintaining cellular membrane integrity in parasites. This disruption leads to cell death in Leishmania species .
- Calcium Homeostasis : The drug significantly affects calcium ion regulation within the parasites. It activates specific calcium channels that increase intracellular calcium levels, disrupting mitochondrial function and leading to apoptosis .
- Immune Modulation : this compound has been shown to upregulate immune markers such as CD16 and CD86 on monocytes while downregulating CD14. This modulation may enhance the host's immune response against Leishmania infections .
Clinical Efficacy
This compound is the first oral medication approved for treating leishmaniasis. Its efficacy has been documented across various studies:
- Visceral Leishmaniasis (VL) : Initial studies reported cure rates of approximately 94% during early trials. However, recent data indicate a decline in efficacy, with cure rates dropping to around 90% over a decade of use .
- Post Kala-Azar Dermal Leishmaniasis (PKDL) : In a study involving 86 patients treated with this compound, 85% achieved clinical cure after 18 months, although relapse rates were notably higher in patients with greater pre-treatment parasite loads .
- Cutaneous Leishmaniasis : A case series demonstrated an overall cure rate of 77% among various Leishmania species treated with this compound. Adverse effects included nausea and vomiting, which were common but manageable .
Case Study 1: Efficacy in PKDL
A five-year study on PKDL patients showed that this compound treatment resulted in an initial cure rate of 78%. However, follow-up revealed a significant relapse rate linked to higher parasite loads at baseline. The study underscores the importance of monitoring parasite susceptibility to this compound over time .
Case Study 2: Cutaneous Leishmaniasis
In a cohort treated for cutaneous leishmaniasis, this compound resulted in an 80% complete response rate. The treatment was well-tolerated overall, although some patients experienced significant side effects requiring dose adjustments .
Resistance Concerns
The emergence of resistance to this compound poses a significant challenge. Studies have shown that parasites isolated from relapsed patients exhibited more than two-fold tolerance to this compound compared to pre-treatment isolates. This highlights the necessity for combination therapies to mitigate resistance risks and improve treatment outcomes .
Summary Table of Efficacy Data
| Condition | Initial Cure Rate | Relapse Rate | Notable Side Effects |
|---|---|---|---|
| Visceral Leishmaniasis | ~94% | ~6.8% | Nausea, vomiting |
| Post Kala-Azar Dermal Leishmaniasis | ~85% | Higher with increased parasite load | Mild gastrointestinal issues |
| Cutaneous Leishmaniasis | ~77% | Not specified | Nausea (97%), lack of appetite (54%) |
Propriétés
IUPAC Name |
hexadecyl 2-(trimethylazaniumyl)ethyl phosphate | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C21H46NO4P/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-20-25-27(23,24)26-21-19-22(2,3)4/h5-21H2,1-4H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
PQLXHQMOHUQAKB-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
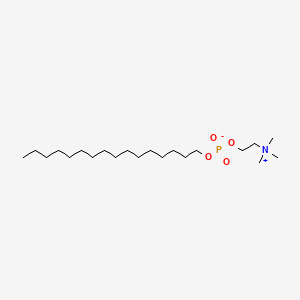
CCCCCCCCCCCCCCCCOP(=O)([O-])OCC[N+](C)(C)C | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C21H46NO4P | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID7045942 | |
| Record name | Miltefosine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7045942 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
407.6 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
Miltefosine has demonstrated activity against Leishmania parasites and neoplastic cells primarily due to its effects on apoptosis and disturbance of lipid-dependent cell signalling pathways. Several potential antileishmanial mechanisms of action have been proposed, however no mechanism has been identified definitely. Within the mitochondria, miltefosine inhibits cytochrome-c oxidase leading to mitochondrial dysfunction and apoptosis-like cell death. Antineoplastic mechanisms of action are related to antileishmanial targets and include inhibition of phosphatidylcholine biosynthesis and inhibition of Akt (also known as protein kinase B), which is a crucial protein within the PI3K/Akt/mTOR intracellular signalling pathway involved in regulating the cell cycle. Animal studies also suggest it may be effective against Trypanosome cruzi (the organism responsible for Chagas' disease), metronidazole-resistant strains of Trichonomas vaginalis, and it may have broad-spectrum anti-fungal activity. | |
| Record name | Miltefosine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09031 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
58066-85-6 | |
| Record name | Miltefosine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=58066-85-6 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Miltefosine [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0058066856 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Miltefosine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB09031 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Miltefosine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=758968 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Miltefosine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID7045942 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Miltefosine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | MILTEFOSINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/53EY29W7EC | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.















