Vincristine
Overview
Description
Vincristine is a vinca alkaloid derived from the Madagascar periwinkle plant, Catharanthus roseus . It is a chemotherapy medication used to treat various types of cancer, including acute lymphocytic leukemia, Hodgkin’s disease, non-Hodgkin’s lymphoma, neuroblastoma, and small cell lung cancer . This compound works by inhibiting cell division, making it a crucial component in cancer treatment regimens .
Preparation Methods
Vincristine is primarily obtained from the natural alkaloid plant source, Catharanthus roseus . The extraction process involves isolating the compound from the plant material, followed by purification steps to obtain the active ingredient . Industrial production methods often involve the use of advanced extraction techniques and purification processes to ensure the compound’s efficacy and safety .
Chemical Reactions Analysis
Vincristine undergoes various chemical reactions, including oxidation, reduction, and substitution . Common reagents used in these reactions include oxidizing agents, reducing agents, and nucleophiles . The major products formed from these reactions depend on the specific conditions and reagents used . For example, oxidation of this compound can lead to the formation of different oxidized derivatives, while reduction can yield reduced forms of the compound .
Scientific Research Applications
Clinical Applications
Vincristine is approved for treating various malignancies, including:
- Acute Lymphoblastic Leukemia (ALL)
- Hodgkin's and Non-Hodgkin Lymphomas
- Neuroblastoma
- Wilms Tumor
- Rhabdomyosarcoma
- Kaposi Sarcoma
FDA-Approved Indications
| Cancer Type | FDA Approval Year |
|---|---|
| Acute Lymphoblastic Leukemia | 1963 |
| Wilms Tumor | 1963 |
| Hodgkin's Lymphoma | 1963 |
| Non-Hodgkin Lymphoma | 1963 |
| Rhabdomyosarcoma | 1963 |
Off-Label Uses
In addition to its approved indications, this compound is used off-label for:
- Central Nervous System (CNS) tumors
- Ewing Sarcoma
- Medulloblastoma
- Bladder Cancer
- Ovarian Cancer
Combination Therapies
This compound is often used in combination with other chemotherapeutic agents to enhance efficacy while minimizing adverse effects. Notable combination regimens include:
- CHOP : Cyclophosphamide, Doxorubicin, this compound, Prednisolone
- CVP : Cyclophosphamide, this compound, Prednisolone
- CISCA : Cisplatin, Doxorubicin, Vinblastine, Bleomycin
Case Studies and Clinical Trials
Numerous studies underscore this compound's effectiveness across different cancer types. A notable trial reported that this compound administered weekly to children with advanced cancer resulted in a 68% objective tumor response rate across various malignancies, including ALL and Hodgkin's disease .
Clinical Trial Insights
| Study Focus | Findings |
|---|---|
| This compound in Advanced Cancer | 68% response rate in pediatric patients |
| Combination Therapy with this compound | Enhanced efficacy in lymphoma treatments |
Pharmacokinetics and Challenges
This compound's pharmacokinetic profile reveals rapid distribution and significant protein binding (approximately 75%). However, its clinical use is limited by neurotoxicity and other side effects such as alopecia and constipation. Recent advancements focus on improving its delivery through nanotechnology-based formulations to enhance targeting and reduce toxicity .
Emerging Trends
Research continues to explore this compound's role in combination therapies and novel formulations. For instance, liposomal formulations like Marqibo have been developed to prolong circulation time and improve dosing efficacy for patients with relapsed ALL . Additionally, ongoing studies are investigating its use in treating solid tumors and enhancing its therapeutic index through innovative drug delivery systems .
Mechanism of Action
Vincristine exerts its effects by binding to tubulin, a protein that is essential for the formation of microtubules . By inhibiting microtubule formation, this compound disrupts the mitotic spindle, leading to cell cycle arrest at the metaphase stage . This inhibition of cell division ultimately results in the death of rapidly dividing cancer cells . Additionally, this compound may interfere with nucleic acid and protein synthesis by blocking the utilization of glutamic acid .
Comparison with Similar Compounds
These compounds share a similar mechanism of action, targeting microtubule formation and inhibiting cell division . vincristine is unique in its specific clinical applications and its relatively lower bone marrow suppression compared to other vinca alkaloids . Vinblastine, for example, is used to treat different types of cancer and has a different toxicity profile . Vindesine and vinflunine are also used in cancer treatment but have distinct pharmacokinetic properties and clinical uses .
Q & A
Basic Research Questions
Q. What experimental models are most effective for studying Vincristine-induced neurotoxicity, and how should variables be controlled?
this compound’s neurotoxic effects are best studied using compartmentalized in vitro models (e.g., microfluidic chambers) to isolate axonal and somatic responses. For example, a concentration of 1 μM this compound applied to axonal compartments induces progressive degeneration, while the same concentration in somatic compartments shows no effect . Key variables to control include exposure duration, drug concentration gradients, and neuronal subtype specificity (e.g., dorsal root ganglion vs. cortical neurons). Include negative controls (e.g., untreated axons) and validate results via morphological (microscopy) and functional (electrophysiology) assays .
Q. What methodologies are recommended for pharmacokinetic analysis of this compound in biological samples?
High-performance liquid chromatography (HPLC) coupled with mass spectrometry (LC-MS/MS) is the gold standard for quantifying this compound in plasma. A vortex-assisted dispersive liquid-liquid microextraction (DLLME) protocol improves recovery rates (≥90%) by optimizing solvent ratios (e.g., chloroform:acetonitrile) and pH conditions (pH 9.0) . Calibration curves should span 0.1–100 ng/mL, with intra-day and inter-day precision maintained at <15% .
Q. How can researchers standardize dosing protocols for this compound in preclinical cancer studies?
Preclinical dosing should align with human equivalent doses (HEDs) calculated via body surface area normalization. For murine models, a typical HED range is 0.5–1 mg/kg administered intravenously weekly. Monitor hematological toxicity (e.g., neutropenia) and neurotoxicity (e.g., gait abnormalities) as endpoints. Use syngeneic or patient-derived xenograft (PDX) models to replicate human pharmacokinetic variability .
Q. What criteria distinguish reliable in vitro efficacy data for this compound across cancer cell lines?
Use cell lines with documented this compound sensitivity (e.g., leukemia Jurkat cells, IC₅₀: 2–5 nM) and resistance (e.g., MCF-7 breast cancer, IC₅₀: >50 nM). Ensure assays include:
- Dose-response curves (6–8 concentrations, 72-hour exposure).
- Proliferation metrics (MTT or ATP-based assays).
- Apoptosis markers (Annexin V/PI flow cytometry). Validate findings with clonogenic assays to assess long-term survival .
Q. How should researchers address batch-to-batch variability in this compound for in vitro studies?
Source this compound from accredited suppliers (e.g., Sigma-Aldrich, Selleckchem) and verify purity (>98%) via certificate of analysis (CoA). Pre-test each batch in a reference cell line (e.g., Jurkat) to confirm IC₅₀ consistency. Normalize data to a positive control (e.g., paclitaxel) to mitigate inter-experimental variability .
Advanced Research Questions
Q. How can high-throughput CRISPR screens identify synergistic drug combinations with this compound?
A CRISPR knockout library (e.g., GeCKO v2) can screen 18 cancer cell lines against 8 drugs, including this compound, to identify gene targets enhancing efficacy. For example, EP300 knockout synergizes with this compound in neuroblastoma models. Use a dual-readout system (cell viability and apoptosis) and validate hits via RNAi or pharmacological inhibition (e.g., JQAD1, an EP300 degrader) . Analyze data with SynergyFinder to quantify combination indices (CI < 0.3 indicates strong synergy) .
Q. What experimental strategies resolve conflicting data on this compound’s efficacy in solid tumors vs. hematological cancers?
Conflicting data often arise from tumor microenvironment (TME) differences. To address this:
- Compare drug penetration using 3D spheroid vs. 2D monolayer cultures.
- Profile ATP-binding cassette (ABC) transporter expression (e.g., P-gp) via qPCR.
- Use intravital imaging to assess this compound distribution in orthotopic models. Apply meta-analysis frameworks (e.g., PRISMA) to harmonize datasets and identify confounding variables .
Q. How can researchers model this compound resistance mechanisms in in vivo systems?
Develop resistance by exposing PDX models to escalating this compound doses over 6–8 weeks. Profile resistance markers via RNA-seq (e.g., upregulated βIII-tubulin, MAPK pathway activation) and validate with CRISPR-Cas9 knockout. Use single-cell sequencing to identify resistant subclones and test combination therapies (e.g., this compound + MAPK inhibitors) .
Q. What advanced imaging techniques optimize this compound delivery studies in the blood-brain barrier (BBB)?
Two-photon microscopy tracks this compound extravasation in real-time using fluorescent analogs (e.g., BODIPY-Vincristine). Combine with dynamic contrast-enhanced MRI to quantify BBB permeability changes in glioblastoma models. Validate delivery efficiency via LC-MS/MS of brain homogenates .
Q. How should multi-omics datasets be analyzed to uncover this compound’s off-target effects?
Integrate transcriptomic (RNA-seq), proteomic (TMT labeling), and metabolomic (LC-MS) data using platforms like MetaboAnalyst or IPA. Focus on pathways enriched in neurotoxicity (e.g., axon guidance, microtubule dynamics) and validate candidates (e.g., MAP1B, CRMP2) via siRNA knockdown. Use machine learning (e.g., random forest) to prioritize biomarkers .
Properties
CAS No. |
57-22-7 |
|---|---|
Molecular Formula |
C46H56N4O10 |
Molecular Weight |
825.0 g/mol |
IUPAC Name |
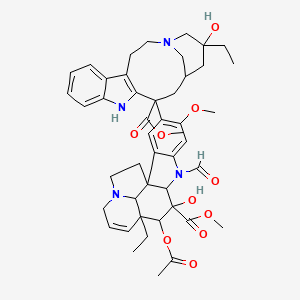
methyl (10S,11R,12R)-11-acetyloxy-12-ethyl-4-[(13S,15S,17S)-17-ethyl-17-hydroxy-13-methoxycarbonyl-1,11-diazatetracyclo[13.3.1.04,12.05,10]nonadeca-4(12),5,7,9-tetraen-13-yl]-8-formyl-10-hydroxy-5-methoxy-8,16-diazapentacyclo[10.6.1.01,9.02,7.016,19]nonadeca-2,4,6,13-tetraene-10-carboxylate |
InChI |
InChI=1S/C46H56N4O10/c1-7-42(55)22-28-23-45(40(53)58-5,36-30(14-18-48(24-28)25-42)29-12-9-10-13-33(29)47-36)32-20-31-34(21-35(32)57-4)50(26-51)38-44(31)16-19-49-17-11-15-43(8-2,37(44)49)39(60-27(3)52)46(38,56)41(54)59-6/h9-13,15,20-21,26,28,37-39,47,55-56H,7-8,14,16-19,22-25H2,1-6H3/t28-,37?,38?,39-,42+,43-,44?,45+,46+/m1/s1 |
InChI Key |
OGWKCGZFUXNPDA-DLBZMDDPSA-N |
impurities |
3'-hydroxyvincristine; 4'-deoxyvincristine; N-desmethylvinblastine; deacetylvincristine; deacetylvinblastine; vinblastine; leurosine; formylleurosine |
SMILES |
CCC1(CC2CC(C3=C(CCN(C2)C1)C4=CC=CC=C4N3)(C5=C(C=C6C(=C5)C78CCN9C7C(C=CC9)(C(C(C8N6C=O)(C(=O)OC)O)OC(=O)C)CC)OC)C(=O)OC)O |
Isomeric SMILES |
CC[C@@]1(C[C@@H]2C[C@@](C3=C(CCN(C2)C1)C4=CC=CC=C4N3)(C5=C(C=C6C(=C5)C78CCN9C7[C@@](C=CC9)([C@H]([C@@](C8N6C=O)(C(=O)OC)O)OC(=O)C)CC)OC)C(=O)OC)O |
Canonical SMILES |
CCC1(CC2CC(C3=C(CCN(C2)C1)C4=CC=CC=C4N3)(C5=C(C=C6C(=C5)C78CCN9C7C(C=CC9)(C(C(C8N6C=O)(C(=O)OC)O)OC(=O)C)CC)OC)C(=O)OC)O |
Appearance |
White to off-white, odorless amorphous or crystalline powder |
Color/Form |
Blades from methanol |
melting_point |
424 to 428 °F (NTP, 1992) 218-220 °C |
Key on ui other cas no. |
57-22-7 |
physical_description |
Vincristine appears as a white crystalline solid. Melting point 218 °C. Used as an antineoplastic. |
shelf_life |
STERILE SOLN IN EITHER H2O OR PHYSIOLOGICAL SALINE STORED IN REFRIGERATOR FOR UP TO 2 WK WITHOUT SIGNIFICANT LOSS OF POTENCY |
solubility |
WHITE TO SLIGHTLY YELLOW, AMORPHOUS OR CRYSTALLINE POWDER; ODORLESS, HYGROSCOPIC; FREELY SOL IN WATER /VINCRISTINE SULFATE USP/ |
Synonyms |
cellcristin Citomid Farmistin Leurocristine Oncovin Oncovine Onkocristin PFS, Vincasar Sulfate, Vincristine Vincasar Vincasar PFS Vincristin Bristol Vincristin medac Vincristine Vincristine Sulfate Vincrisul Vintec |
Origin of Product |
United States |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.
















