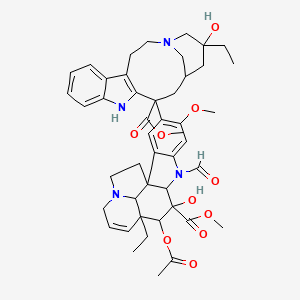
Vincristine
- Cliquez sur DEMANDE RAPIDE pour recevoir un devis de notre équipe d'experts.
- Avec des produits de qualité à un prix COMPÉTITIF, vous pouvez vous concentrer davantage sur votre recherche.
Vue d'ensemble
Description
Vincristine is a vinca alkaloid derived from the Madagascar periwinkle plant, Catharanthus roseus . It is a chemotherapy medication used to treat various types of cancer, including acute lymphocytic leukemia, Hodgkin’s disease, non-Hodgkin’s lymphoma, neuroblastoma, and small cell lung cancer . This compound works by inhibiting cell division, making it a crucial component in cancer treatment regimens .
Méthodes De Préparation
Vincristine is primarily obtained from the natural alkaloid plant source, Catharanthus roseus . The extraction process involves isolating the compound from the plant material, followed by purification steps to obtain the active ingredient . Industrial production methods often involve the use of advanced extraction techniques and purification processes to ensure the compound’s efficacy and safety .
Analyse Des Réactions Chimiques
Vincristine undergoes various chemical reactions, including oxidation, reduction, and substitution . Common reagents used in these reactions include oxidizing agents, reducing agents, and nucleophiles . The major products formed from these reactions depend on the specific conditions and reagents used . For example, oxidation of this compound can lead to the formation of different oxidized derivatives, while reduction can yield reduced forms of the compound .
Applications De Recherche Scientifique
Clinical Applications
Vincristine is approved for treating various malignancies, including:
- Acute Lymphoblastic Leukemia (ALL)
- Hodgkin's and Non-Hodgkin Lymphomas
- Neuroblastoma
- Wilms Tumor
- Rhabdomyosarcoma
- Kaposi Sarcoma
FDA-Approved Indications
| Cancer Type | FDA Approval Year |
|---|---|
| Acute Lymphoblastic Leukemia | 1963 |
| Wilms Tumor | 1963 |
| Hodgkin's Lymphoma | 1963 |
| Non-Hodgkin Lymphoma | 1963 |
| Rhabdomyosarcoma | 1963 |
Off-Label Uses
In addition to its approved indications, this compound is used off-label for:
- Central Nervous System (CNS) tumors
- Ewing Sarcoma
- Medulloblastoma
- Bladder Cancer
- Ovarian Cancer
Combination Therapies
This compound is often used in combination with other chemotherapeutic agents to enhance efficacy while minimizing adverse effects. Notable combination regimens include:
- CHOP : Cyclophosphamide, Doxorubicin, this compound, Prednisolone
- CVP : Cyclophosphamide, this compound, Prednisolone
- CISCA : Cisplatin, Doxorubicin, Vinblastine, Bleomycin
Case Studies and Clinical Trials
Numerous studies underscore this compound's effectiveness across different cancer types. A notable trial reported that this compound administered weekly to children with advanced cancer resulted in a 68% objective tumor response rate across various malignancies, including ALL and Hodgkin's disease .
Clinical Trial Insights
| Study Focus | Findings |
|---|---|
| This compound in Advanced Cancer | 68% response rate in pediatric patients |
| Combination Therapy with this compound | Enhanced efficacy in lymphoma treatments |
Pharmacokinetics and Challenges
This compound's pharmacokinetic profile reveals rapid distribution and significant protein binding (approximately 75%). However, its clinical use is limited by neurotoxicity and other side effects such as alopecia and constipation. Recent advancements focus on improving its delivery through nanotechnology-based formulations to enhance targeting and reduce toxicity .
Emerging Trends
Research continues to explore this compound's role in combination therapies and novel formulations. For instance, liposomal formulations like Marqibo have been developed to prolong circulation time and improve dosing efficacy for patients with relapsed ALL . Additionally, ongoing studies are investigating its use in treating solid tumors and enhancing its therapeutic index through innovative drug delivery systems .
Mécanisme D'action
Vincristine exerts its effects by binding to tubulin, a protein that is essential for the formation of microtubules . By inhibiting microtubule formation, this compound disrupts the mitotic spindle, leading to cell cycle arrest at the metaphase stage . This inhibition of cell division ultimately results in the death of rapidly dividing cancer cells . Additionally, this compound may interfere with nucleic acid and protein synthesis by blocking the utilization of glutamic acid .
Comparaison Avec Des Composés Similaires
These compounds share a similar mechanism of action, targeting microtubule formation and inhibiting cell division . vincristine is unique in its specific clinical applications and its relatively lower bone marrow suppression compared to other vinca alkaloids . Vinblastine, for example, is used to treat different types of cancer and has a different toxicity profile . Vindesine and vinflunine are also used in cancer treatment but have distinct pharmacokinetic properties and clinical uses .
Propriétés
Numéro CAS |
57-22-7 |
|---|---|
Formule moléculaire |
C46H56N4O10 |
Poids moléculaire |
825.0 g/mol |
Nom IUPAC |
methyl (10S,11R,12R)-11-acetyloxy-12-ethyl-4-[(13S,15S,17S)-17-ethyl-17-hydroxy-13-methoxycarbonyl-1,11-diazatetracyclo[13.3.1.04,12.05,10]nonadeca-4(12),5,7,9-tetraen-13-yl]-8-formyl-10-hydroxy-5-methoxy-8,16-diazapentacyclo[10.6.1.01,9.02,7.016,19]nonadeca-2,4,6,13-tetraene-10-carboxylate |
InChI |
InChI=1S/C46H56N4O10/c1-7-42(55)22-28-23-45(40(53)58-5,36-30(14-18-48(24-28)25-42)29-12-9-10-13-33(29)47-36)32-20-31-34(21-35(32)57-4)50(26-51)38-44(31)16-19-49-17-11-15-43(8-2,37(44)49)39(60-27(3)52)46(38,56)41(54)59-6/h9-13,15,20-21,26,28,37-39,47,55-56H,7-8,14,16-19,22-25H2,1-6H3/t28-,37?,38?,39-,42+,43-,44?,45+,46+/m1/s1 |
Clé InChI |
OGWKCGZFUXNPDA-DLBZMDDPSA-N |
Impuretés |
3'-hydroxyvincristine; 4'-deoxyvincristine; N-desmethylvinblastine; deacetylvincristine; deacetylvinblastine; vinblastine; leurosine; formylleurosine |
SMILES |
CCC1(CC2CC(C3=C(CCN(C2)C1)C4=CC=CC=C4N3)(C5=C(C=C6C(=C5)C78CCN9C7C(C=CC9)(C(C(C8N6C=O)(C(=O)OC)O)OC(=O)C)CC)OC)C(=O)OC)O |
SMILES isomérique |
CC[C@@]1(C[C@@H]2C[C@@](C3=C(CCN(C2)C1)C4=CC=CC=C4N3)(C5=C(C=C6C(=C5)C78CCN9C7[C@@](C=CC9)([C@H]([C@@](C8N6C=O)(C(=O)OC)O)OC(=O)C)CC)OC)C(=O)OC)O |
SMILES canonique |
CCC1(CC2CC(C3=C(CCN(C2)C1)C4=CC=CC=C4N3)(C5=C(C=C6C(=C5)C78CCN9C7C(C=CC9)(C(C(C8N6C=O)(C(=O)OC)O)OC(=O)C)CC)OC)C(=O)OC)O |
Apparence |
White to off-white, odorless amorphous or crystalline powder |
Color/Form |
Blades from methanol |
melting_point |
424 to 428 °F (NTP, 1992) 218-220 °C |
Key on ui other cas no. |
57-22-7 |
Description physique |
Vincristine appears as a white crystalline solid. Melting point 218 °C. Used as an antineoplastic. |
Durée de conservation |
STERILE SOLN IN EITHER H2O OR PHYSIOLOGICAL SALINE STORED IN REFRIGERATOR FOR UP TO 2 WK WITHOUT SIGNIFICANT LOSS OF POTENCY |
Solubilité |
WHITE TO SLIGHTLY YELLOW, AMORPHOUS OR CRYSTALLINE POWDER; ODORLESS, HYGROSCOPIC; FREELY SOL IN WATER /VINCRISTINE SULFATE USP/ |
Synonymes |
cellcristin Citomid Farmistin Leurocristine Oncovin Oncovine Onkocristin PFS, Vincasar Sulfate, Vincristine Vincasar Vincasar PFS Vincristin Bristol Vincristin medac Vincristine Vincristine Sulfate Vincrisul Vintec |
Origine du produit |
United States |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.
















