Dexpropranolol
Descripción general
Descripción
Dexpropranolol es una molécula pequeña que pertenece a la clase de compuestos orgánicos conocidos como naftalenos. Es un enantiómero del propranolol, un conocido antagonista no selectivo de los receptores beta-adrenérgicos. This compound se utiliza experimentalmente y ha mostrado potencial en diversas aplicaciones farmacológicas .
Mecanismo De Acción
Dexpropranolol ejerce sus efectos bloqueando los receptores beta-adrenérgicos, que están involucrados en la respuesta a las catecolaminas como la adrenalina y la noradrenalina. Este bloqueo conduce a una disminución de la frecuencia cardíaca, la presión arterial y la demanda de oxígeno miocárdico. Los objetivos moleculares incluyen los receptores adrenérgicos beta-1 y beta-2, y las vías involucradas están relacionadas con la inhibición de la producción de AMP cíclico y la posterior señalización descendente .
Análisis Bioquímico
Biochemical Properties
Dexpropranolol plays a role in biochemical reactions primarily as a beta-adrenergic receptor antagonist. It interacts with beta-adrenergic receptors, inhibiting the action of catecholamines such as epinephrine and norepinephrine . This interaction reduces the heart rate and blood pressure, making it useful in treating conditions like hypertension and arrhythmias . This compound also interacts with other biomolecules, including enzymes and proteins involved in signal transduction pathways .
Cellular Effects
This compound affects various types of cells and cellular processes. It influences cell function by modulating cell signaling pathways, particularly those involving beta-adrenergic receptors . This modulation can lead to changes in gene expression and cellular metabolism. For example, this compound can reduce the production of cyclic AMP (cAMP), a secondary messenger involved in many cellular processes . This reduction in cAMP levels can affect processes such as glycogenolysis and lipolysis .
Molecular Mechanism
The molecular mechanism of this compound involves its binding to beta-adrenergic receptors, preventing the activation of these receptors by catecholamines . This inhibition reduces the downstream effects of receptor activation, such as the activation of adenylate cyclase and the subsequent production of cAMP . By reducing cAMP levels, this compound can inhibit various cellular processes, including those involved in energy metabolism and muscle contraction .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of this compound can change over time. The stability and degradation of this compound can influence its long-term effects on cellular function . Studies have shown that this compound can maintain its activity over extended periods, but its effectiveness may decrease due to degradation . Long-term exposure to this compound can lead to adaptive changes in cells, such as receptor desensitization and changes in gene expression .
Dosage Effects in Animal Models
The effects of this compound vary with different dosages in animal models. At low doses, this compound can effectively reduce heart rate and blood pressure without significant adverse effects . At high doses, this compound can cause toxic effects, including bradycardia, hypotension, and respiratory depression . Threshold effects have been observed, where the therapeutic effects plateau at higher doses, and adverse effects become more pronounced .
Metabolic Pathways
This compound is involved in various metabolic pathways, including those related to its metabolism and elimination . It is primarily metabolized in the liver by cytochrome P450 enzymes, particularly CYP2D6 . The metabolites of this compound are then excreted in the urine . This compound can also affect metabolic flux and metabolite levels by inhibiting beta-adrenergic receptors, which play a role in regulating energy metabolism .
Transport and Distribution
This compound is transported and distributed within cells and tissues through various mechanisms . It can cross cell membranes and bind to intracellular receptors, affecting its localization and accumulation . This compound can also interact with transporters and binding proteins that facilitate its movement within the body . These interactions can influence the distribution and effectiveness of this compound in different tissues .
Subcellular Localization
The subcellular localization of this compound can affect its activity and function . This compound can be found in various cellular compartments, including the cytoplasm and cell membrane . It may also be localized to specific organelles, such as the endoplasmic reticulum and mitochondria . The localization of this compound can be influenced by targeting signals and post-translational modifications that direct it to specific compartments .
Métodos De Preparación
Dexpropranolol puede sintetizarse a través de varias rutas sintéticas. Un método común implica la resolución del propranolol racèmico utilizando agentes quirales para separar el enantiómero dextrorrotatorio. Las condiciones de reacción típicamente implican el uso de disolventes como el dimetilsulfóxido (DMSO) y catalizadores quirales específicos . Los métodos de producción industrial pueden implicar procesos de resolución a gran escala y técnicas de purificación para garantizar la alta pureza enantiomérica del this compound.
Análisis De Reacciones Químicas
Dexpropranolol experimenta diversas reacciones químicas, incluyendo:
Oxidación: Puede oxidarse utilizando reactivos como el permanganato de potasio o el trióxido de cromo para formar naftoquinonas correspondientes.
Reducción: Las reacciones de reducción pueden llevarse a cabo utilizando gas hidrógeno en presencia de paladio sobre carbono para obtener derivados reducidos.
Los principales productos formados a partir de estas reacciones dependen de los reactivos y condiciones específicos utilizados.
Aplicaciones Científicas De Investigación
Pharmacological Profile
Dexpropranolol exhibits local anesthetic properties similar to those of propranolol but with negligible beta-adrenergic receptor blocking activity. This distinct profile allows for potential applications in areas where traditional beta-blockers may not be suitable.
Key Characteristics of this compound
| Property | Description |
|---|---|
| Type | Beta-adrenergic antagonist |
| Isomer | Dextro isomer of propranolol |
| Local Anesthetic Action | Present, similar to propranolol |
| Beta-Blockade | Negligible |
Clinical Applications
-
Cardiovascular Conditions
- This compound has been studied in the context of angina pectoris and exercise tolerance. A comparative study indicated that while this compound did not significantly affect exercise time, propranolol and practolol improved exercise tolerance in patients with angina . This suggests that this compound may not be as effective as its racemic counterpart in managing certain cardiovascular symptoms.
- Migraine Prophylaxis
-
Psychological Effects
- Emerging studies suggest that propranolol can influence emotional responses and implicit biases, raising questions about whether this compound could have similar effects without the associated beta-blocking properties. Research indicates that propranolol reduces implicit racial bias, potentially due to its effects on the autonomic nervous system . Further exploration into this compound's impact on psychological conditions could yield valuable insights.
- Post-Traumatic Stress Disorder (PTSD)
Case Study 1: Exercise Tolerance in Angina Patients
A study comparing this compound with racemic propranolol and practolol revealed that while both propranolol and practolol improved exercise tolerance, this compound did not show significant effects. This highlights the importance of understanding the specific actions of each isomer in clinical settings .
Case Study 2: Psychological Impact
In a study examining the effects of propranolol on implicit racial bias, participants receiving propranolol scored lower on measures of subconscious bias compared to those receiving a placebo. This raises questions about whether this compound could modulate such biases without the cardiovascular implications of traditional beta-blockers .
Comparación Con Compuestos Similares
Dexpropranolol es similar a otros antagonistas de los receptores beta-adrenérgicos, como:
Propranolol: La mezcla racémica de la que se deriva el this compound.
Atenolol: Un antagonista selectivo del receptor beta-1 adrenérgico utilizado principalmente para afecciones cardiovasculares.
Metoprolol: Otro bloqueador beta-1 selectivo con aplicaciones similares al atenolol.
La singularidad de this compound reside en su estereoquímica específica, que puede dar lugar a diferentes propiedades farmacocinéticas y farmacodinámicas en comparación con su contraparte racémica .
Actividad Biológica
Dexpropranolol is a selective beta-adrenergic antagonist derived from propranolol, primarily used in the management of various cardiovascular conditions. This article explores the biological activity of this compound, including its pharmacokinetics, mechanisms of action, therapeutic applications, and safety profile, supported by data tables and relevant case studies.
Pharmacokinetics
This compound exhibits distinct pharmacokinetic properties compared to its racemic counterpart, propranolol. Key pharmacokinetic parameters include:
This compound is cleared more rapidly than propranolol, primarily because it does not significantly affect hepatic blood flow, leading to a more predictable pharmacokinetic profile .
This compound functions by selectively blocking beta-adrenergic receptors, particularly β1 receptors in the heart. This blockade results in:
- Reduced heart rate : By inhibiting the effects of catecholamines on the heart.
- Decreased myocardial contractility : Leading to lower oxygen demand during stress.
- Vasodilation : Although less pronounced than with other beta-blockers.
These actions contribute to its therapeutic efficacy in managing hypertension and preventing angina pectoris .
Therapeutic Applications
This compound has been studied for various clinical applications:
- Hypertension : Clinical trials have demonstrated its effectiveness in lowering blood pressure compared to placebo and other antihypertensives.
- Anxiety Disorders : It is used off-label for performance anxiety due to its ability to mitigate physical symptoms like tachycardia and tremors.
- Migraine Prophylaxis : Some studies suggest it may help reduce the frequency of migraine attacks.
A meta-analysis indicated that this compound significantly reduces the risk of disease progression in infants with retinopathy of prematurity (ROP), showing a relative risk (RR) of 0.65 for stage progression compared to controls .
Safety Profile
While generally well-tolerated, this compound can cause adverse effects similar to other beta-blockers:
| Adverse Effect | Incidence Rate |
|---|---|
| Bradycardia | 11.42 RR [95% CI, 0.66–196.40] |
| Hypotension | 7.27 RR [95% CI, 0.39–133.95] |
| Hypoglycemia | 3.10 RR [95% CI, 0.33–29.27] |
The increased risk of adverse events was noted in a meta-analysis involving infants treated with propranolol, indicating a need for careful monitoring during treatment .
Case Studies
Several case studies highlight the clinical efficacy of this compound:
- Case Study 1 : A 30-year-old male with generalized anxiety disorder reported significant improvement in symptoms after initiating treatment with this compound, particularly during public speaking engagements.
- Case Study 2 : An infant diagnosed with ROP showed marked improvement in disease stage after receiving this compound, reducing the need for laser therapy.
Propiedades
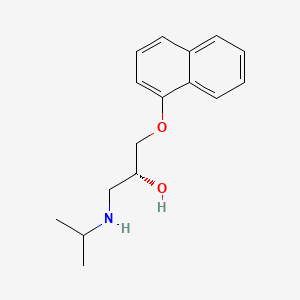
IUPAC Name |
(2R)-1-naphthalen-1-yloxy-3-(propan-2-ylamino)propan-2-ol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C16H21NO2/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16/h3-9,12,14,17-18H,10-11H2,1-2H3/t14-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
AQHHHDLHHXJYJD-CQSZACIVSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)NCC(COC1=CC=CC2=CC=CC=C21)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC(C)NC[C@H](COC1=CC=CC2=CC=CC=C21)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C16H21NO2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID3045304 | |
| Record name | Dexpropranolol | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3045304 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
259.34 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
5051-22-9, 13071-11-9 | |
| Record name | (+)-Propranolol | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=5051-22-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Dexpropranolol [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0005051229 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Dexpropranolol | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB03322 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Dexpropranolol | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3045304 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Dexpropranolol | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.023.409 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | (R)-[2-hydroxy-3-(naphthyloxy)propyl]isopropylammonium chloride | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.032.677 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | DEXPROPRANOLOL | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/PG6KY07UD7 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Synthesis routes and methods I
Procedure details








Synthesis routes and methods II
Procedure details





Synthesis routes and methods III
Procedure details







Synthesis routes and methods IV
Procedure details




Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.













