デキソプロプラノロール
概要
説明
デクスプロプラノロールは、ナフタレン類として知られる有機化合物のクラスに属する低分子です。これは、よく知られている非選択的βアドレナリン受容体拮抗薬であるプロプラノロールの立体異性体です。 デクスプロプラノロールは実験的に使用されており、さまざまな薬理学的用途で可能性を示しています .
科学的研究の応用
Pharmacological Profile
Dexpropranolol exhibits local anesthetic properties similar to those of propranolol but with negligible beta-adrenergic receptor blocking activity. This distinct profile allows for potential applications in areas where traditional beta-blockers may not be suitable.
Key Characteristics of Dexpropranolol
| Property | Description |
|---|---|
| Type | Beta-adrenergic antagonist |
| Isomer | Dextro isomer of propranolol |
| Local Anesthetic Action | Present, similar to propranolol |
| Beta-Blockade | Negligible |
Clinical Applications
-
Cardiovascular Conditions
- Dexpropranolol has been studied in the context of angina pectoris and exercise tolerance. A comparative study indicated that while dexpropranolol did not significantly affect exercise time, propranolol and practolol improved exercise tolerance in patients with angina . This suggests that dexpropranolol may not be as effective as its racemic counterpart in managing certain cardiovascular symptoms.
- Migraine Prophylaxis
-
Psychological Effects
- Emerging studies suggest that propranolol can influence emotional responses and implicit biases, raising questions about whether dexpropranolol could have similar effects without the associated beta-blocking properties. Research indicates that propranolol reduces implicit racial bias, potentially due to its effects on the autonomic nervous system . Further exploration into dexpropranolol's impact on psychological conditions could yield valuable insights.
- Post-Traumatic Stress Disorder (PTSD)
Case Study 1: Exercise Tolerance in Angina Patients
A study comparing dexpropranolol with racemic propranolol and practolol revealed that while both propranolol and practolol improved exercise tolerance, dexpropranolol did not show significant effects. This highlights the importance of understanding the specific actions of each isomer in clinical settings .
Case Study 2: Psychological Impact
In a study examining the effects of propranolol on implicit racial bias, participants receiving propranolol scored lower on measures of subconscious bias compared to those receiving a placebo. This raises questions about whether dexpropranolol could modulate such biases without the cardiovascular implications of traditional beta-blockers .
作用機序
デクスプロプラノロールは、アドレナリンやノルアドレナリンなどのカテコールアミンへの応答に関与するβアドレナリン受容体を遮断することにより、その効果を発揮します。この遮断は、心拍数、血圧、心筋酸素要求量の低下につながります。 分子標的はβ1およびβ2アドレナリン受容体であり、関与する経路は環状AMP産生の阻害とそれに続く下流シグナル伝達に関連しています .
生化学分析
Biochemical Properties
Dexpropranolol plays a role in biochemical reactions primarily as a beta-adrenergic receptor antagonist. It interacts with beta-adrenergic receptors, inhibiting the action of catecholamines such as epinephrine and norepinephrine . This interaction reduces the heart rate and blood pressure, making it useful in treating conditions like hypertension and arrhythmias . Dexpropranolol also interacts with other biomolecules, including enzymes and proteins involved in signal transduction pathways .
Cellular Effects
Dexpropranolol affects various types of cells and cellular processes. It influences cell function by modulating cell signaling pathways, particularly those involving beta-adrenergic receptors . This modulation can lead to changes in gene expression and cellular metabolism. For example, dexpropranolol can reduce the production of cyclic AMP (cAMP), a secondary messenger involved in many cellular processes . This reduction in cAMP levels can affect processes such as glycogenolysis and lipolysis .
Molecular Mechanism
The molecular mechanism of dexpropranolol involves its binding to beta-adrenergic receptors, preventing the activation of these receptors by catecholamines . This inhibition reduces the downstream effects of receptor activation, such as the activation of adenylate cyclase and the subsequent production of cAMP . By reducing cAMP levels, dexpropranolol can inhibit various cellular processes, including those involved in energy metabolism and muscle contraction .
Temporal Effects in Laboratory Settings
In laboratory settings, the effects of dexpropranolol can change over time. The stability and degradation of dexpropranolol can influence its long-term effects on cellular function . Studies have shown that dexpropranolol can maintain its activity over extended periods, but its effectiveness may decrease due to degradation . Long-term exposure to dexpropranolol can lead to adaptive changes in cells, such as receptor desensitization and changes in gene expression .
Dosage Effects in Animal Models
The effects of dexpropranolol vary with different dosages in animal models. At low doses, dexpropranolol can effectively reduce heart rate and blood pressure without significant adverse effects . At high doses, dexpropranolol can cause toxic effects, including bradycardia, hypotension, and respiratory depression . Threshold effects have been observed, where the therapeutic effects plateau at higher doses, and adverse effects become more pronounced .
Metabolic Pathways
Dexpropranolol is involved in various metabolic pathways, including those related to its metabolism and elimination . It is primarily metabolized in the liver by cytochrome P450 enzymes, particularly CYP2D6 . The metabolites of dexpropranolol are then excreted in the urine . Dexpropranolol can also affect metabolic flux and metabolite levels by inhibiting beta-adrenergic receptors, which play a role in regulating energy metabolism .
Transport and Distribution
Dexpropranolol is transported and distributed within cells and tissues through various mechanisms . It can cross cell membranes and bind to intracellular receptors, affecting its localization and accumulation . Dexpropranolol can also interact with transporters and binding proteins that facilitate its movement within the body . These interactions can influence the distribution and effectiveness of dexpropranolol in different tissues .
Subcellular Localization
The subcellular localization of dexpropranolol can affect its activity and function . Dexpropranolol can be found in various cellular compartments, including the cytoplasm and cell membrane . It may also be localized to specific organelles, such as the endoplasmic reticulum and mitochondria . The localization of dexpropranolol can be influenced by targeting signals and post-translational modifications that direct it to specific compartments .
準備方法
デクスプロプラノロールは、いくつかの合成経路を通じて合成することができます。一般的な方法の1つは、キラル剤を用いてラセミ体プロプラノロールを分割し、右旋性エナンチオマーを分離することです。 反応条件は通常、ジメチルスルホキシド(DMSO)などの溶媒と特定のキラル触媒の使用を伴います . 工業生産方法は、デクスプロプラノロールの高いエナンチオマー純度を保証するために、大規模な分割プロセスと精製技術を伴う場合があります。
化学反応の分析
デクスプロプラノロールは、次のようなさまざまな化学反応を受けます。
酸化: 過マンガン酸カリウムまたは三酸化クロムなどの試薬を使用して酸化して、対応するナフトキノンを形成できます。
還元: 還元反応は、炭素上のパラジウムの存在下で水素ガスを使用して、還元された誘導体を生成できます。
置換: ナフタレン環で求核置換反応が起こることがあり、多くの場合、水素化ナトリウムやハロアルカンなどの試薬を使用します.
これらの反応から生成される主要な生成物は、使用される特定の試薬と条件によって異なります。
科学研究アプリケーション
化学: これは、他の複雑な分子の合成におけるキラルビルディングブロックとして使用されます。
生物学: 研究では、生物系に対するその影響、特にβアドレナリン受容体との相互作用が調査されています。
医学: デクスプロプラノロールは、心臓血管疾患、不安障害、およびβブロッカーが有効なその他の状態の治療に有望視されています.
類似化合物との比較
デクスプロプラノロールは、次のような他のβアドレナリン受容体拮抗薬に似ています。
プロプラノロール: デクスプロプラノロールが由来するラセミ体混合物。
アテノロール: 主に心臓血管疾患に使用される選択的β1アドレナリン受容体拮抗薬。
メトプロロール: アテノロールと同様の用途を持つ別の選択的β1ブロッカー。
デクスプロプラノロールの独自性は、その特定の立体化学にあり、これはラセミ体対応物と比較して、異なる薬物動態的および薬力学的特性をもたらす可能性があります .
生物活性
Dexpropranolol is a selective beta-adrenergic antagonist derived from propranolol, primarily used in the management of various cardiovascular conditions. This article explores the biological activity of dexpropranolol, including its pharmacokinetics, mechanisms of action, therapeutic applications, and safety profile, supported by data tables and relevant case studies.
Pharmacokinetics
Dexpropranolol exhibits distinct pharmacokinetic properties compared to its racemic counterpart, propranolol. Key pharmacokinetic parameters include:
Dexpropranolol is cleared more rapidly than propranolol, primarily because it does not significantly affect hepatic blood flow, leading to a more predictable pharmacokinetic profile .
Dexpropranolol functions by selectively blocking beta-adrenergic receptors, particularly β1 receptors in the heart. This blockade results in:
- Reduced heart rate : By inhibiting the effects of catecholamines on the heart.
- Decreased myocardial contractility : Leading to lower oxygen demand during stress.
- Vasodilation : Although less pronounced than with other beta-blockers.
These actions contribute to its therapeutic efficacy in managing hypertension and preventing angina pectoris .
Therapeutic Applications
Dexpropranolol has been studied for various clinical applications:
- Hypertension : Clinical trials have demonstrated its effectiveness in lowering blood pressure compared to placebo and other antihypertensives.
- Anxiety Disorders : It is used off-label for performance anxiety due to its ability to mitigate physical symptoms like tachycardia and tremors.
- Migraine Prophylaxis : Some studies suggest it may help reduce the frequency of migraine attacks.
A meta-analysis indicated that dexpropranolol significantly reduces the risk of disease progression in infants with retinopathy of prematurity (ROP), showing a relative risk (RR) of 0.65 for stage progression compared to controls .
Safety Profile
While generally well-tolerated, dexpropranolol can cause adverse effects similar to other beta-blockers:
| Adverse Effect | Incidence Rate |
|---|---|
| Bradycardia | 11.42 RR [95% CI, 0.66–196.40] |
| Hypotension | 7.27 RR [95% CI, 0.39–133.95] |
| Hypoglycemia | 3.10 RR [95% CI, 0.33–29.27] |
The increased risk of adverse events was noted in a meta-analysis involving infants treated with propranolol, indicating a need for careful monitoring during treatment .
Case Studies
Several case studies highlight the clinical efficacy of dexpropranolol:
- Case Study 1 : A 30-year-old male with generalized anxiety disorder reported significant improvement in symptoms after initiating treatment with dexpropranolol, particularly during public speaking engagements.
- Case Study 2 : An infant diagnosed with ROP showed marked improvement in disease stage after receiving dexpropranolol, reducing the need for laser therapy.
特性
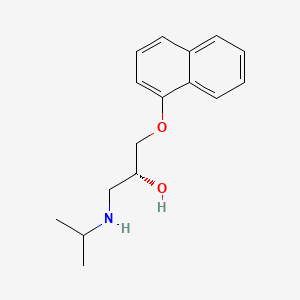
IUPAC Name |
(2R)-1-naphthalen-1-yloxy-3-(propan-2-ylamino)propan-2-ol | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C16H21NO2/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16/h3-9,12,14,17-18H,10-11H2,1-2H3/t14-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
AQHHHDLHHXJYJD-CQSZACIVSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CC(C)NCC(COC1=CC=CC2=CC=CC=C21)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
CC(C)NC[C@H](COC1=CC=CC2=CC=CC=C21)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C16H21NO2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID3045304 | |
| Record name | Dexpropranolol | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3045304 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
259.34 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
5051-22-9, 13071-11-9 | |
| Record name | (+)-Propranolol | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=5051-22-9 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Dexpropranolol [INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0005051229 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Dexpropranolol | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB03322 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Dexpropranolol | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID3045304 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Dexpropranolol | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.023.409 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | (R)-[2-hydroxy-3-(naphthyloxy)propyl]isopropylammonium chloride | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.032.677 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | DEXPROPRANOLOL | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/PG6KY07UD7 | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Synthesis routes and methods I
Procedure details








Synthesis routes and methods II
Procedure details





Synthesis routes and methods III
Procedure details







Synthesis routes and methods IV
Procedure details




Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。













