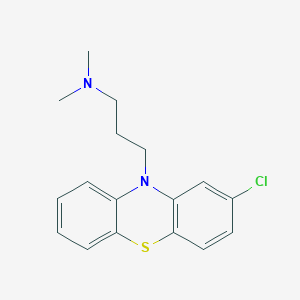
Chlorpromazine
Descripción general
Descripción
La clorpromazina es un potente fármaco tranquilizante sintético que actúa selectivamente sobre los centros superiores del cerebro como un depresor del sistema nervioso central. Se utiliza principalmente para tratar trastornos psicóticos como la esquizofrenia, el trastorno bipolar, problemas de comportamiento graves en niños, náuseas y vómitos, ansiedad antes de la cirugía e hipo persistente . La clorpromazina se sintetizó por primera vez en 1950 y se puso a disposición del público para uso médico a mediados de la década de 1950 .
Métodos De Preparación
La clorpromazina se sintetiza mediante un proceso químico de varios pasos. La síntesis normalmente implica la reacción de 2-clorofenoxtiazina con N,N-dimetil-1,3-propandiamina en condiciones específicas . Los métodos de producción industrial a menudo implican el uso de varios disolventes y catalizadores para optimizar el rendimiento y la pureza. El compuesto es sensible a la luz y se oscurece fácilmente cuando se expone .
Análisis De Reacciones Químicas
La clorpromazina sufre varios tipos de reacciones químicas, incluida la oxidación, la reducción y la sustitución. Los reactivos comunes utilizados en estas reacciones incluyen azul de bromofenol, tampón citrato y varios ácidos y bases . Los principales productos formados a partir de estas reacciones incluyen varios metabolitos, que se excretan en la orina . El compuesto también puede sufrir reacciones fotoquímicas, lo que lleva a la formación de radicales y otras especies reactivas .
Aplicaciones Científicas De Investigación
Psychiatric Applications
1. Schizophrenia Treatment
Chlorpromazine is primarily indicated for the treatment of schizophrenia, particularly effective in managing positive symptoms such as hallucinations and delusions. Studies have demonstrated its efficacy in reducing these symptoms, leading to improved patient outcomes .
2. Bipolar Disorder
In bipolar disorder, this compound is used to manage acute manic episodes. It helps control symptoms like excessive energy, decreased need for sleep, and impulsivity . Clinical trials have shown that this compound can stabilize mood and reduce the severity of manic episodes .
3. Acute Psychosis
this compound is effective in treating acute psychosis, including agitation and aggressive behavior in children. It has been shown to provide rapid control of symptoms in emergency settings .
Medical Applications
1. Nausea and Vomiting
this compound is also used to control nausea and vomiting, particularly in postoperative settings or for patients undergoing chemotherapy. Its antiemetic properties make it a valuable option for managing these symptoms .
2. Persistent Singultus (Hiccups)
The FDA has approved this compound for treating persistent hiccups that last more than 48 hours. This application is particularly beneficial for patients with chronic hiccups that do not respond to other treatments .
3. Adjunct Treatment in Tetanus
this compound can be used as an adjunct treatment for tetanus, helping to manage muscle spasms associated with the condition .
Emerging Applications
1. Neuroprotection in Stroke
Recent studies have explored the potential of this compound as a neuroprotective agent in acute ischemic stroke (AIS). A clinical trial (RICHES) is investigating its safety and efficacy when administered intravenously alongside promethazine shortly after stroke onset. Preliminary findings suggest it may reduce infarct volume and neurological deficits .
2. Management of Serotonin Syndrome
this compound has been utilized off-label for managing serotonin syndrome, a potentially life-threatening condition resulting from excessive serotonergic activity in the nervous system .
Case Studies
| Application | Study/Trial | Findings |
|---|---|---|
| Schizophrenia | Meta-analysis of multiple studies | Significant reduction in positive symptoms |
| Bipolar Disorder | Randomized controlled trials | Effective in stabilizing manic episodes |
| Acute Psychosis | Emergency department case reports | Rapid symptom control observed |
| Stroke Neuroprotection | RICHES trial (ongoing) | Preliminary evidence of reduced infarct volume |
| Persistent Hiccups | Clinical observations | Successful management after 48 hours |
Mecanismo De Acción
El mecanismo de acción preciso de la clorpromazina no está del todo claro, pero se cree que está relacionado con su capacidad para bloquear los receptores de dopamina en el cerebro . La clorpromazina inhibe los receptores D2 de dopamina, lo que da como resultado una reducción de la transmisión dopaminérgica dentro de las cuatro vías dopaminérgicas . También inhibe los receptores de serotonina (5-HT), muscarínicos y α1-adrenérgicos . Estas acciones contribuyen a sus efectos antipsicóticos, sedantes y antieméticos .
Comparación Con Compuestos Similares
La clorpromazina pertenece a la clase de fármacos antipsicóticos de las fenotiazinas. Compuestos similares incluyen tioridazina, flufenazina y trifluoperazina . En comparación con estos compuestos, la clorpromazina tiene una gama más amplia de usos terapéuticos y un perfil de efectos secundarios único. Por ejemplo, tiene una mayor afinidad por los receptores D1 en comparación con otras fenotiazinas . Esto la hace particularmente eficaz en el tratamiento de una amplia gama de trastornos psicóticos y otras afecciones.
Propiedades
IUPAC Name |
3-(2-chlorophenothiazin-10-yl)-N,N-dimethylpropan-1-amine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
ZPEIMTDSQAKGNT-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN(C)CCCN1C2=CC=CC=C2SC3=C1C=C(C=C3)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C17H19ClN2S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID0022808 | |
| Record name | Chlorpromazine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0022808 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
318.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Chlorpromazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014620 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Boiling Point |
200-205 °C at 8.00E-01 mm Hg, BP: 200-205 °C at 0.8 mm Hg | |
| Record name | Chlorpromazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00477 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Chlorpromazine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3033 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Solubility |
Very soluble in ethanol, ether, benzene and chloroform; soluble in dilute hydrochloric acid, In water, 2.55X10-3 g/L (2.55 mg/L) at 24 °C, 4.17e-03 g/L | |
| Record name | Chlorpromazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00477 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Chlorpromazine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3033 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Chlorpromazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014620 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Chlorpromazine acts as an antagonist (blocking agent) on different postsysnaptic receptors -on dopaminergic-receptors (subtypes D1, D2, D3 and D4 - different antipsychotic properties on productive and unproductive symptoms), on serotonergic-receptors (5-HT1 and 5-HT2, with anxiolytic, antidepressive and antiaggressive properties as well as an attenuation of extrapypramidal side-effects, but also leading to weight gain, fall in blood pressure, sedation and ejaculation difficulties), on histaminergic-receptors (H1-receptors, sedation, antiemesis, vertigo, fall in blood pressure and weight gain), alpha1/alpha2-receptors (antisympathomimetic properties, lowering of blood pressure, reflex tachycardia, vertigo, sedation, hypersalivation and incontinence as well as sexual dysfunction, but may also attenuate pseudoparkinsonism - controversial) and finally on muscarinic (cholinergic) M1/M2-receptors (causing anticholinergic symptoms like dry mouth, blurred vision, obstipation, difficulty/inability to urinate, sinus tachycardia, ECG-changes and loss of memory, but the anticholinergic action may attenuate extrapyramidal side-effects). Additionally, Chlorpromazine is a weak presynaptic inhibitor of Dopamine reuptake, which may lead to (mild) antidepressive and antiparkinsonian effects. This action could also account for psychomotor agitation and amplification of psychosis (very rarely noted in clinical use)., The principal pharmacologic effects of chlorpromazine are similar to those of other propylamino derivatives of phenothiazine. Chlorpromazine has strong anticholinergic and sedative effects and moderate extrapyramidal effects. Chlorpromazine has strong antiemetic and adrenergic blocking activity and weak ganglionic blocking, antihistaminic, and antiserotonergic activity., The development of phenothiazine derivatives as psychopharmacologic agents resulted from the observation that certain phenothiazine antihistaminic compounds produced sedation. In an attempt to enhance the sedative effects of these drugs, promethazine and chlorpromazine were synthesized. Chlorpromazine is the pharmacologic prototype of the phenothiazines. The pharmacology of phenothiazines is complex, and because of their actions on the central and autonomic nervous systems, the drugs affect many different sites in the body. Although the actions of the various phenothiazines are generally similar, these drugs differ both quantitatively and qualitatively in the extent to which they produce specific pharmacologic effects. /Phenothiazine General Statement/, In the CNS, phenothiazines act principally at the subcortical levels of the reticular formation, limbic system, and hypothalamus. Phenothiazines generally do not produce substantial cortical depression; however, there is minimal information on the specific effects of phenothiazines at the cortical level. Phenothiazines also act in the basal ganglia, exhibiting extrapyramidal effects. The precise mechanism(s) of action, including antipsychotic action, of phenothiazines has not been determined, but may be principally related to antidopaminergic effects of the drugs. There is evidence to indicate that phenothiazines antagonize dopamine-mediated neurotransmission at the synapses. There is also some evidence that phenothiazines may block postsynaptic dopamine receptor sites. However, it has not been determined whether the antipsychotic effect of the drugs is causally related to their antidopaminergic effects. Phenothiazines also have peripheral and/or central antagonistic activity against alpha-adrenergic, serotonergic, histaminic (H1-receptors), and muscarinic receptors. Phenothiazines also have some adrenergic activity, since they block the reuptake of monoamines at the presynaptic neuronal membrane, which tends to enhance neurotransmission. The effects of phenothiazines on the autonomic nervous system are complex and unpredictable because the drugs exhibit varying degrees of alpha-adrenergic blocking, muscarinic blocking, and adrenergic activity. The antipsychotic activity of phenothiazines may be related to any or all of these effects, but it has been suggested that the drugs' effects on dopamine are probably most important. It has also been suggested that effects of phenothiazines on other amines (eg, gamma-aminobutyric acid [GABA]) or peptides (eg, substance P, endorphins) may contribute to their antipsychotic effect. Further study is needed to determine the role of central neuronal receptor antagonism and of effects on biochemical mediators in the antipsychotic action of the phenothiazines and other antipsychotic agents. /Phenothiazine General Statement/, Although the exact mechanism(s) of action has not been conclusively determined, phenothiazines have an antiemetic effect. The antiemetic activity may be mediated via a direct effect of the drugs on the medullary chemoreceptor trigger zone (CTZ), apparently by blocking dopamine receptors in the CTZ. Phenothiazines inhibit the central and peripheral effects of apomorphine and ergot alkaloids. Phenothiazines generally do not inhibit emesis caused by the action of drugs at the nodose ganglion or by local action on the GI tract. /Phenothiazine General Statement/, For more Mechanism of Action (Complete) data for Chlorpromazine (19 total), please visit the HSDB record page. | |
| Record name | Chlorpromazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00477 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Chlorpromazine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3033 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Oily liquid, White, crystalline solid | |
CAS No. |
34468-21-8, 50-53-3 | |
| Record name | 10H-Phenothiazine-10-propanamine, 2-chloro-N,N-dimethyl-, radical ion(1+) | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=34468-21-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Chlorpromazine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=50-53-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Chlorpromazine [USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000050533 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Chlorpromazine cation radical | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0034468218 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Chlorpromazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00477 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | chlorpromazine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=756689 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | chlorpromazine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=167745 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Chlorpromazine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0022808 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Chlorpromazine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.042 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CHLORPROMAZINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/U42B7VYA4P | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Chlorpromazine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3033 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Chlorpromazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014620 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
177-178, About 60 °C, < 25 °C | |
| Record name | Chlorpromazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00477 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Chlorpromazine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3033 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Chlorpromazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014620 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details




Synthesis routes and methods II
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.













