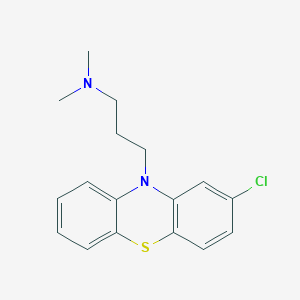
Chlorpromazine
概述
描述
氯丙嗪是一种有效的合成镇静剂,作为中枢神经系统的抑制剂,选择性作用于大脑的高级中枢。 它主要用于治疗精神疾病,如精神分裂症、双相情感障碍、儿童严重的行为问题、恶心和呕吐、手术前的焦虑和顽固性呃逆 。 氯丙嗪于 1950 年首次合成,并在 1950 年代中期开始广泛用于医疗领域 .
准备方法
氯丙嗪通过多步化学合成过程合成。 该合成通常涉及在特定条件下使 2-氯吩噻嗪与 N,N-二甲基-1,3-丙二胺反应 。工业生产方法通常涉及使用各种溶剂和催化剂来优化产量和纯度。 该化合物对光敏感,暴露后很容易变黑 .
化学反应分析
氯丙嗪会发生多种类型的化学反应,包括氧化、还原和取代。 这些反应中常用的试剂包括溴酚蓝、柠檬酸盐缓冲液以及各种酸和碱 。 这些反应的主要产物包括各种代谢物,这些代谢物会通过尿液排出 。 该化合物还会发生光化学反应,导致形成自由基和其他反应性物质 .
科学研究应用
Psychiatric Applications
1. Schizophrenia Treatment
Chlorpromazine is primarily indicated for the treatment of schizophrenia, particularly effective in managing positive symptoms such as hallucinations and delusions. Studies have demonstrated its efficacy in reducing these symptoms, leading to improved patient outcomes .
2. Bipolar Disorder
In bipolar disorder, this compound is used to manage acute manic episodes. It helps control symptoms like excessive energy, decreased need for sleep, and impulsivity . Clinical trials have shown that this compound can stabilize mood and reduce the severity of manic episodes .
3. Acute Psychosis
this compound is effective in treating acute psychosis, including agitation and aggressive behavior in children. It has been shown to provide rapid control of symptoms in emergency settings .
Medical Applications
1. Nausea and Vomiting
this compound is also used to control nausea and vomiting, particularly in postoperative settings or for patients undergoing chemotherapy. Its antiemetic properties make it a valuable option for managing these symptoms .
2. Persistent Singultus (Hiccups)
The FDA has approved this compound for treating persistent hiccups that last more than 48 hours. This application is particularly beneficial for patients with chronic hiccups that do not respond to other treatments .
3. Adjunct Treatment in Tetanus
this compound can be used as an adjunct treatment for tetanus, helping to manage muscle spasms associated with the condition .
Emerging Applications
1. Neuroprotection in Stroke
Recent studies have explored the potential of this compound as a neuroprotective agent in acute ischemic stroke (AIS). A clinical trial (RICHES) is investigating its safety and efficacy when administered intravenously alongside promethazine shortly after stroke onset. Preliminary findings suggest it may reduce infarct volume and neurological deficits .
2. Management of Serotonin Syndrome
this compound has been utilized off-label for managing serotonin syndrome, a potentially life-threatening condition resulting from excessive serotonergic activity in the nervous system .
Case Studies
| Application | Study/Trial | Findings |
|---|---|---|
| Schizophrenia | Meta-analysis of multiple studies | Significant reduction in positive symptoms |
| Bipolar Disorder | Randomized controlled trials | Effective in stabilizing manic episodes |
| Acute Psychosis | Emergency department case reports | Rapid symptom control observed |
| Stroke Neuroprotection | RICHES trial (ongoing) | Preliminary evidence of reduced infarct volume |
| Persistent Hiccups | Clinical observations | Successful management after 48 hours |
作用机制
相似化合物的比较
氯丙嗪属于吩噻嗪类抗精神病药物。 类似的化合物包括硫利达嗪、氟奋乃静和三氟拉嗪 。与这些化合物相比,氯丙嗪具有更广泛的治疗用途和独特的副作用谱。 例如,与其他吩噻嗪类药物相比,它对 D1 受体的亲和力更高 。这使得它在治疗各种精神疾病和其他疾病方面特别有效。
属性
IUPAC Name |
3-(2-chlorophenothiazin-10-yl)-N,N-dimethylpropan-1-amine | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
ZPEIMTDSQAKGNT-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CN(C)CCCN1C2=CC=CC=C2SC3=C1C=C(C=C3)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C17H19ClN2S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID0022808 | |
| Record name | Chlorpromazine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0022808 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
318.9 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Solid | |
| Record name | Chlorpromazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014620 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Boiling Point |
200-205 °C at 8.00E-01 mm Hg, BP: 200-205 °C at 0.8 mm Hg | |
| Record name | Chlorpromazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00477 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Chlorpromazine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3033 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Solubility |
Very soluble in ethanol, ether, benzene and chloroform; soluble in dilute hydrochloric acid, In water, 2.55X10-3 g/L (2.55 mg/L) at 24 °C, 4.17e-03 g/L | |
| Record name | Chlorpromazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00477 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Chlorpromazine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3033 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Chlorpromazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014620 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Chlorpromazine acts as an antagonist (blocking agent) on different postsysnaptic receptors -on dopaminergic-receptors (subtypes D1, D2, D3 and D4 - different antipsychotic properties on productive and unproductive symptoms), on serotonergic-receptors (5-HT1 and 5-HT2, with anxiolytic, antidepressive and antiaggressive properties as well as an attenuation of extrapypramidal side-effects, but also leading to weight gain, fall in blood pressure, sedation and ejaculation difficulties), on histaminergic-receptors (H1-receptors, sedation, antiemesis, vertigo, fall in blood pressure and weight gain), alpha1/alpha2-receptors (antisympathomimetic properties, lowering of blood pressure, reflex tachycardia, vertigo, sedation, hypersalivation and incontinence as well as sexual dysfunction, but may also attenuate pseudoparkinsonism - controversial) and finally on muscarinic (cholinergic) M1/M2-receptors (causing anticholinergic symptoms like dry mouth, blurred vision, obstipation, difficulty/inability to urinate, sinus tachycardia, ECG-changes and loss of memory, but the anticholinergic action may attenuate extrapyramidal side-effects). Additionally, Chlorpromazine is a weak presynaptic inhibitor of Dopamine reuptake, which may lead to (mild) antidepressive and antiparkinsonian effects. This action could also account for psychomotor agitation and amplification of psychosis (very rarely noted in clinical use)., The principal pharmacologic effects of chlorpromazine are similar to those of other propylamino derivatives of phenothiazine. Chlorpromazine has strong anticholinergic and sedative effects and moderate extrapyramidal effects. Chlorpromazine has strong antiemetic and adrenergic blocking activity and weak ganglionic blocking, antihistaminic, and antiserotonergic activity., The development of phenothiazine derivatives as psychopharmacologic agents resulted from the observation that certain phenothiazine antihistaminic compounds produced sedation. In an attempt to enhance the sedative effects of these drugs, promethazine and chlorpromazine were synthesized. Chlorpromazine is the pharmacologic prototype of the phenothiazines. The pharmacology of phenothiazines is complex, and because of their actions on the central and autonomic nervous systems, the drugs affect many different sites in the body. Although the actions of the various phenothiazines are generally similar, these drugs differ both quantitatively and qualitatively in the extent to which they produce specific pharmacologic effects. /Phenothiazine General Statement/, In the CNS, phenothiazines act principally at the subcortical levels of the reticular formation, limbic system, and hypothalamus. Phenothiazines generally do not produce substantial cortical depression; however, there is minimal information on the specific effects of phenothiazines at the cortical level. Phenothiazines also act in the basal ganglia, exhibiting extrapyramidal effects. The precise mechanism(s) of action, including antipsychotic action, of phenothiazines has not been determined, but may be principally related to antidopaminergic effects of the drugs. There is evidence to indicate that phenothiazines antagonize dopamine-mediated neurotransmission at the synapses. There is also some evidence that phenothiazines may block postsynaptic dopamine receptor sites. However, it has not been determined whether the antipsychotic effect of the drugs is causally related to their antidopaminergic effects. Phenothiazines also have peripheral and/or central antagonistic activity against alpha-adrenergic, serotonergic, histaminic (H1-receptors), and muscarinic receptors. Phenothiazines also have some adrenergic activity, since they block the reuptake of monoamines at the presynaptic neuronal membrane, which tends to enhance neurotransmission. The effects of phenothiazines on the autonomic nervous system are complex and unpredictable because the drugs exhibit varying degrees of alpha-adrenergic blocking, muscarinic blocking, and adrenergic activity. The antipsychotic activity of phenothiazines may be related to any or all of these effects, but it has been suggested that the drugs' effects on dopamine are probably most important. It has also been suggested that effects of phenothiazines on other amines (eg, gamma-aminobutyric acid [GABA]) or peptides (eg, substance P, endorphins) may contribute to their antipsychotic effect. Further study is needed to determine the role of central neuronal receptor antagonism and of effects on biochemical mediators in the antipsychotic action of the phenothiazines and other antipsychotic agents. /Phenothiazine General Statement/, Although the exact mechanism(s) of action has not been conclusively determined, phenothiazines have an antiemetic effect. The antiemetic activity may be mediated via a direct effect of the drugs on the medullary chemoreceptor trigger zone (CTZ), apparently by blocking dopamine receptors in the CTZ. Phenothiazines inhibit the central and peripheral effects of apomorphine and ergot alkaloids. Phenothiazines generally do not inhibit emesis caused by the action of drugs at the nodose ganglion or by local action on the GI tract. /Phenothiazine General Statement/, For more Mechanism of Action (Complete) data for Chlorpromazine (19 total), please visit the HSDB record page. | |
| Record name | Chlorpromazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00477 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Chlorpromazine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3033 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Oily liquid, White, crystalline solid | |
CAS No. |
34468-21-8, 50-53-3 | |
| Record name | 10H-Phenothiazine-10-propanamine, 2-chloro-N,N-dimethyl-, radical ion(1+) | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=34468-21-8 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Chlorpromazine | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=50-53-3 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Chlorpromazine [USP:INN:BAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000050533 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Chlorpromazine cation radical | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0034468218 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Chlorpromazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00477 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | chlorpromazine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=756689 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | chlorpromazine | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=167745 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Chlorpromazine | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID0022808 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Chlorpromazine | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.042 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CHLORPROMAZINE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/U42B7VYA4P | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | Chlorpromazine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3033 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Chlorpromazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014620 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
177-178, About 60 °C, < 25 °C | |
| Record name | Chlorpromazine | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00477 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Chlorpromazine | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/3033 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Chlorpromazine | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014620 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods I
Procedure details




Synthesis routes and methods II
Procedure details





Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。














