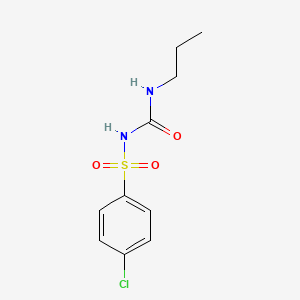
Chlorpropamide
Descripción general
Descripción
La clorpropamida es un fármaco antidiabético que pertenece a la clase de compuestos orgánicos de las sulfonilureas. Se utiliza principalmente para tratar la diabetes mellitus tipo 2 mediante la estimulación de la liberación de insulina de las células beta pancreáticas. La clorpropamida es una sulfonilurea de primera generación de acción prolongada y es conocida por su capacidad para mantener los niveles de glucosa en sangre durante un período prolongado .
Métodos De Preparación
Rutas sintéticas y condiciones de reacción
La clorpropamida se puede sintetizar mediante un proceso de varios pasos que implica la reacción de cloruro de 4-clorobencenosulfonilo con propilamina para formar 4-clorobencenosulfonamida. Este intermedio se hace reaccionar luego con fosgeno y amoníaco para producir clorpropamida. Las condiciones de reacción suelen implicar temperaturas controladas y el uso de disolventes como el diclorometano .
Métodos de producción industrial
La producción industrial de clorpropamida sigue rutas sintéticas similares pero a mayor escala. El proceso implica el uso de reactores grandes y un control preciso de las condiciones de reacción para garantizar un alto rendimiento y pureza. El producto final se somete a rigurosas medidas de control de calidad para cumplir con los estándares farmacéuticos .
Análisis De Reacciones Químicas
Tipos de reacciones
La clorpropamida experimenta varios tipos de reacciones químicas, que incluyen:
Oxidación: La clorpropamida se puede oxidar para formar sulfoxidos y sulfonas.
Reducción: Las reacciones de reducción pueden convertir la clorpropamida en sus derivados de amina correspondientes.
Sustitución: La clorpropamida puede sufrir reacciones de sustitución nucleofílica, particularmente en el grupo sulfonilo
Reactivos y condiciones comunes
Oxidación: Los agentes oxidantes comunes incluyen peróxido de hidrógeno y permanganato de potasio.
Reducción: Se utilizan agentes reductores como hidruro de aluminio y litio y borohidruro de sodio.
Sustitución: Los nucleófilos como las aminas y los tioles pueden reaccionar con la clorpropamida en condiciones suaves
Principales productos formados
Oxidación: Sulfoxidos y sulfonas.
Reducción: Derivados de amina.
Sustitución: Varias sulfonamidas sustituidas
Aplicaciones Científicas De Investigación
Chlorpropamide is an antidiabetic drug in the sulfonylurea class, used to manage diabetes mellitus type 2 . It is an oral antihyperglycemic agent used for treating non-insulin-dependent diabetes mellitus . this compound stimulates the pancreas' β cells to release insulin, increasing both basal insulin secretion and meal-stimulated insulin release .
Clinical Applications in Diabetes Management
This compound is primarily used to lower blood glucose levels in conjunction with diet in patients with type II diabetes mellitus . Studies have demonstrated its effectiveness in controlling blood sugar levels:
- A six-year study involving 479 patients indicated that 58% (196 out of 339) achieved satisfactory blood sugar control with this compound .
- In a study comparing this compound to tolbutamide, this compound was more effective in reducing fasting blood sugar levels . 83% of patients responded positively to this compound, compared to 60% for tolbutamide .
- This compound has shown a lower primary failure rate compared to glibenclamide in long-term trials .
Case Study: Long-Term Use of this compound
A study published in JAMA observed 479 patients over six years, with 339 patients completing the analysis. The findings indicated that 58% of patients achieved satisfactory glycemic control . However, 42% experienced unsatisfactory control, including 31 patients with primary and 17 with secondary failure. Notably, 95 patients had previously shown unsatisfactory responses to other sulfonylureas .
Adverse Effects:
- Gastrointestinal issues (9 patients)
- Skin rash (7 patients)
- Symptomatic hypoglycemia (4 patients)
- Jaundice due to this compound hypersensitivity (1 patient)
Off-Label Use in Diabetes Insipidus
This compound is also used off-label to manage vasopressin-sensitive diabetes insipidus . It appears that this compound needs some level of endogenous antidiuretic hormone (ADH) to be present to produce an antidiuresis . this compound may enhance the effect of submaximal levels of endogenous ADH, leading to an antidiuretic effect .
Case Study: this compound in Diabetes Insipidus
In a study of 13 patients with vasopressin-sensitive diabetes insipidus, 10 experienced an antidiuresis with this compound treatment . The drug's antidiuretic action is similar to that of Pitressin, decreasing "free" water clearance without altering creatinine excretion . The effect of exogenous Pitressin was also potentiated when patients were pretreated with this compound, suggesting that this compound enhances the action of existing ADH in patients with diabetes insipidus .
Potential Adverse Effects
While this compound is effective, it is associated with several potential adverse effects :
- Hypoglycemia: Risk is increased in elderly, debilitated, and malnourished individuals . Consistent food intake is necessary to mitigate this risk .
- Gastrointestinal Issues: Including nausea and diarrhea .
- Skin Reactions: Such as rash .
- Cholestatic Jaundice: A case study reported cholestatic jaundice and pseudomembranous colitis in a 59-year-old man treated with this compound .
Drug Interactions and Metabolism
Mecanismo De Acción
La clorpropamida ejerce sus efectos uniéndose a los canales de potasio sensibles al ATP en la superficie de la célula beta pancreática. Esta unión reduce la conductancia del potasio, lo que provoca la despolarización de la membrana celular. La despolarización desencadena la apertura de los canales de calcio activados por voltaje, lo que conduce a una afluencia de iones calcio. El aumento de la concentración intracelular de calcio estimula la exocitosis de los gránulos que contienen insulina, lo que aumenta la secreción de insulina .
Comparación Con Compuestos Similares
La clorpropamida se compara con otras sulfonilureas como:
- Gliclazida
- Tolbutamida
- Glipizida
Unicidad
La clorpropamida es única debido a su larga duración de acción y su capacidad para mantener los niveles de glucosa en sangre estables durante un período prolongado. Tiene un mayor riesgo de causar hipoglucemia en comparación con las sulfonilureas de acción más corta como la gliclazida y la tolbutamida .
Compuestos similares
- Gliclazida : Sulfonilurea de acción más corta con un menor riesgo de hipoglucemia.
- Tolbutamida : Otra sulfonilurea de primera generación con una duración de acción más corta.
- Glipizida : Sulfonilurea de segunda generación con un perfil de efectos secundarios más favorable .
Actividad Biológica
Chlorpropamide is a second-generation sulfonylurea antidiabetic agent primarily used in the management of type 2 diabetes mellitus. Its biological activity is characterized by its ability to stimulate insulin secretion from pancreatic beta cells, thereby lowering blood glucose levels. This article delves into the mechanisms of action, pharmacokinetics, clinical efficacy, and associated side effects of this compound, supported by relevant case studies and research findings.
This compound acts by binding to ATP-sensitive potassium channels on the surface of pancreatic beta cells. This binding reduces potassium conductance, leading to membrane depolarization. The depolarization triggers an influx of calcium ions through voltage-sensitive calcium channels, which subsequently stimulates the exocytosis of insulin. Additionally, this compound enhances peripheral glucose utilization and decreases hepatic gluconeogenesis, contributing to its hypoglycemic effect .
Pharmacokinetics
- Absorption : this compound is readily absorbed from the gastrointestinal tract, with peak plasma concentrations occurring within 2-4 hours post-administration. The onset of action is typically within one hour, and the maximal effect is observed between 3-6 hours after ingestion .
- Distribution : It is highly bound to plasma proteins, with a half-life averaging approximately 36 hours (ranging from 25 to 60 hours) and a duration of effect lasting at least 24 hours .
- Metabolism and Excretion : About 80% of this compound is metabolized in the liver into various metabolites, including 2-hydroxylthis compound and p-chlorobenzenesulfonylurea. The drug and its metabolites are primarily excreted through urine within 96 hours .
Clinical Efficacy
A long-term study involving 479 patients demonstrated that this compound effectively controlled blood glucose levels in approximately 58% of participants over a six-year period. Among those who had previously shown unsatisfactory responses to other sulfonylureas, this compound proved beneficial for many .
Case Study: Hyponatremia Risk
Research indicated that this compound treatment was associated with an incidence of hyponatremia (low serum sodium levels) in about 6.3% of patients. This condition was notably higher compared to patients treated with other sulfonylureas such as tolbutamide or glibenclamide. Elderly patients and those on thiazide diuretics were identified as having increased risk factors for this adverse effect .
Side Effects
While generally well-tolerated, this compound can cause several side effects:
- Hypoglycemia : A common risk associated with sulfonylureas, hypoglycemia can occur especially in elderly or malnourished individuals .
- Gastrointestinal Issues : Reports indicated gastrointestinal disturbances in some patients during long-term therapy .
- Skin Reactions : Skin rashes were observed in a subset of patients .
- Jaundice : Cases of jaundice linked to hypersensitivity reactions have been documented .
Comparative Efficacy
In a comparative study between this compound and tolbutamide, this compound demonstrated superior efficacy in lowering fasting blood sugar levels. It was successful in achieving satisfactory control in 83% of cases compared to 60% for tolbutamide. Additionally, this compound required lower maximum dosages among responders .
Summary Table of Key Findings
| Parameter | This compound | Tolbutamide |
|---|---|---|
| Success Rate | 83% | 60% |
| Common Side Effects | Hypoglycemia, Jaundice | Less frequent |
| Risk of Hyponatremia | Higher (6.3%) | Lower (0.6%) |
| Half-Life | ~36 hours | ~8 hours |
Q & A
Basic Research Questions
Q. What are the validated analytical methods for quantifying chlorpropamide in pharmaceutical formulations, and how do their parameters compare?
The British Pharmacopeia recommends spectrophotometry, while the United States Pharmacopeia adopts HPLC . A spectrophotometric method using chloranilic acid (π-acceptor) forms a violet complex (λmax 530 nm) with this compound, validated for linearity (5–25 µg/mL), accuracy (recovery studies), and precision (RSD <2%) . HPLC offers higher specificity for complex matrices but requires costly instrumentation. Researchers should prioritize method selection based on laboratory resources and required sensitivity .
Q. How can researchers design reproducible clinical studies to assess this compound-alcohol flushing (CPAF) responses?
Key considerations include:
- Blinding : Double-blind, placebo-controlled crossover designs (e.g., this compound vs. placebo administered one week apart) to minimize bias .
- Exclusion criteria : Participants on NSAIDs (e.g., aspirin) or other drugs that may block flushing responses must be excluded .
- Standardized questionnaires : Avoid leading questions; instead, use symptom lists (e.g., "flushing of the face") to capture self-reported responses objectively .
- Compliance monitoring : Address attrition rates (e.g., 34–37% non-completion in diabetic cohorts) through reminders and simplified protocols .
Q. What statistical approaches are suitable for analyzing this compound efficacy data in heterogeneous diabetic populations?
Use Yates’s χ² test for categorical outcomes (e.g., CPAF incidence) and unpaired Student’s t-test for continuous variables (e.g., HbA1c reduction). For longitudinal data, mixed-effects models can account for repeated measures and dropout biases .
Advanced Research Questions
Q. How can researchers resolve discrepancies in this compound’s pharmacokinetic data across studies, particularly regarding plasma half-life variability?
Confounding factors include:
- Genetic polymorphisms : CYP2C9 enzyme variants alter this compound metabolism, affecting half-life (reported range: 24–42 hrs) .
- Drug interactions : Co-administration with NSAIDs or sulfonamides may displace this compound from plasma proteins, altering free concentrations . Mitigation strategies:
- Conduct in vitro hepatic microsome assays to identify metabolic pathways.
- Use population pharmacokinetic modeling to quantify inter-individual variability .
Q. What experimental frameworks optimize this compound assay robustness in biological matrices (e.g., plasma)?
- Sample pretreatment : Protein precipitation with acetonitrile or solid-phase extraction to reduce matrix interference .
- Validation parameters : Include selectivity (against endogenous compounds), recovery (>95%), and stability under storage conditions .
- Cross-validation : Compare results with LC-MS/MS for confirmation .
Q. How does this compound induce SIADH (syndrome of inappropriate antidiuretic hormone secretion), and what mechanistic studies are needed?
this compound enhances renal tubular sensitivity to ADH by upregulating aquaporin-2 channels and increasing Na<sup>+</sup>/Cl<sup>−</sup> reabsorption in the loop of Henle . Unresolved questions:
- Dose dependency : Is SIADH risk correlated with this compound dosage or duration?
- Genetic susceptibility : Do polymorphisms in ADH receptor genes (e.g., AVPR2) modulate this effect? Proposed methodology:
- In vitro models: Use collecting duct cell lines to quantify ADH receptor expression post-exposure.
- In vivo studies**: Measure urinary osmolality and serum sodium in patients on long-term therapy .
Q. What strategies improve the reproducibility of this compound’s polymorphic transformation studies during tablet formulation?
- In situ monitoring : Employ mechanical Raman spectroscopy to track crystal structure changes during compression .
- Environmental controls : Regulate humidity and temperature to prevent hydrate formation.
- Excipient screening : Test stabilizers (e.g., cellulose derivatives) to inhibit polymorphic shifts .
Q. Methodological Guidance
- Literature Review : Prioritize primary sources (e.g., Journal of Scientific Research) over unverified databases .
- Ethical Compliance : Obtain IRB approval for human studies; exclude vulnerable populations (e.g., pregnant women) unless benefits outweigh risks .
- Data Reporting : Use FAIR principles (Findable, Accessible, Interoperable, Reusable) for supplementary materials .
Propiedades
IUPAC Name |
1-(4-chlorophenyl)sulfonyl-3-propylurea | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C10H13ClN2O3S/c1-2-7-12-10(14)13-17(15,16)9-5-3-8(11)4-6-9/h3-6H,2,7H2,1H3,(H2,12,13,14) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
RKWGIWYCVPQPMF-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
CCCNC(=O)NS(=O)(=O)C1=CC=C(C=C1)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C10H13ClN2O3S | |
| Record name | CHLOROPROPAMIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20013 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID9020322 | |
| Record name | Chlorpropamide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID9020322 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
276.74 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Physical Description |
Chloropropamide is a white crystalline powder with a slight odor. (NTP, 1992), Solid | |
| Record name | CHLOROPROPAMIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20013 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Chlorpropamide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014810 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Solubility |
>41.5 [ug/mL] (The mean of the results at pH 7.4), less than 1 mg/mL at 57 °F (NTP, 1992), 2.2 mg/ml in water @ pH 6; practically insol in water @ pH 7.3; sol in alcohol; moderately sol in chloroform; sparingly sol in ether, benzene, In water, 258 mg/l @ 37 °C, 1.57e-01 g/L | |
| Record name | SID855559 | |
| Source | Burnham Center for Chemical Genomics | |
| URL | https://pubchem.ncbi.nlm.nih.gov/bioassay/1996#section=Data-Table | |
| Description | Aqueous solubility in buffer at pH 7.4 | |
| Record name | CHLOROPROPAMIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20013 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Chlorpropamide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00672 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | CHLORPROPAMIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/2051 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Chlorpropamide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014810 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Mechanism of Action |
Sulfonylureas such as chlorpropamide bind to ATP-sensitive potassium channels on the pancreatic cell surface, reducing potassium conductance and causing depolarization of the membrane. Depolarization stimulates calcium ion influx through voltage-sensitive calcium channels, raising intracellular concentrations of calcium ions, which induces the secretion, or exocytosis, of insulin., ...ACTION OF SULFONYLUREAS APPEARS TO BE STIMULATION OF RELEASE OF INSULIN FROM BETA CELLS. ...TO BE EFFECTIVE, PT MUST HAVE SOME FUNCTIONAL ISLET CELLS... /HYPOGLYCEMIC SULFONYLUREAS/, Sulfonylureas cause hypoglycemia by stimulating insulin release from pancreatic beta cells. Their effects in the treatment of diabetes ... are more complex. /Sulfonylureas/, Sulfonylureas are now...thought to act by a number of different mechanisms. 1. ...produce a depolarization of the pancreatic islet beta cell membrane potassium ion permeability. This results in a release of preformed insulin into the circulation and occurs mostly in non-insulin dependent diabetics. 2. ...reduce basal glucose output from the liver... 3. increase insulin receptor binding... 4. ...increasing intracellular levels of AMP... 5. increase insulin secretion by suppressing the release of glucagon and somatostatin from alpha and delta pancreatic cells. /Sulfonylureas/, Sulfonylureas lower blood glucose in NIDDM by directly stimulating the acute release of insulin from functioning beta cells of pancreatic islet tissue by an unknown process that involves a sulfonylurea receptor on the beta cell. Sulfonylureas inhibit the ATP potassium channels on the beta cell membrane and potassium efflux, which results in depolarization and calcium influx, calcium-calmodulin binding, kinase activation, and release of insulin containing granules by exocytosis, an effect similar to that of glucose. Insulin is a hormone that lowers blood glucose and controls the storage and metabolism of carbohydrates, proteins, and fats. Therefore, sulfonylureas are effective only in patients whose pancreata are capable of producing insulin. /Sulfonylurea antidiabetic agents/ | |
| Record name | Chlorpropamide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00672 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | CHLORPROPAMIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/2051 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
Color/Form |
Crystals from dil ethanol, WHITE, CRYSTALLINE POWDER, White, crystalline powder | |
CAS No. |
94-20-2 | |
| Record name | CHLOROPROPAMIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20013 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Chlorpropamide | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=94-20-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Chlorpropamide [USP:INN:BAN:JAN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0000094202 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Chlorpropamide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00672 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | chlorpropamide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=756690 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | chlorpropamide | |
| Source | DTP/NCI | |
| URL | https://dtp.cancer.gov/dtpstandard/servlet/dwindex?searchtype=NSC&outputformat=html&searchlist=44634 | |
| Description | The NCI Development Therapeutics Program (DTP) provides services and resources to the academic and private-sector research communities worldwide to facilitate the discovery and development of new cancer therapeutic agents. | |
| Explanation | Unless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source. | |
| Record name | Chlorpropamide | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID9020322 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Chlorpropamide | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/substance-information/-/substanceinfo/100.002.104 | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | CHLORPROPAMIDE | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/WTM2C3IL2X | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
| Record name | CHLORPROPAMIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/2051 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Chlorpropamide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014810 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Melting Point |
261 to 264 °F (NTP, 1992), 129.2-129.8, 127-129 °C, 127 - 129 °C | |
| Record name | CHLOROPROPAMIDE | |
| Source | CAMEO Chemicals | |
| URL | https://cameochemicals.noaa.gov/chemical/20013 | |
| Description | CAMEO Chemicals is a chemical database designed for people who are involved in hazardous material incident response and planning. CAMEO Chemicals contains a library with thousands of datasheets containing response-related information and recommendations for hazardous materials that are commonly transported, used, or stored in the United States. CAMEO Chemicals was developed by the National Oceanic and Atmospheric Administration's Office of Response and Restoration in partnership with the Environmental Protection Agency's Office of Emergency Management. | |
| Explanation | CAMEO Chemicals and all other CAMEO products are available at no charge to those organizations and individuals (recipients) responsible for the safe handling of chemicals. However, some of the chemical data itself is subject to the copyright restrictions of the companies or organizations that provided the data. | |
| Record name | Chlorpropamide | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB00672 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | CHLORPROPAMIDE | |
| Source | Hazardous Substances Data Bank (HSDB) | |
| URL | https://pubchem.ncbi.nlm.nih.gov/source/hsdb/2051 | |
| Description | The Hazardous Substances Data Bank (HSDB) is a toxicology database that focuses on the toxicology of potentially hazardous chemicals. It provides information on human exposure, industrial hygiene, emergency handling procedures, environmental fate, regulatory requirements, nanomaterials, and related areas. The information in HSDB has been assessed by a Scientific Review Panel. | |
| Record name | Chlorpropamide | |
| Source | Human Metabolome Database (HMDB) | |
| URL | http://www.hmdb.ca/metabolites/HMDB0014810 | |
| Description | The Human Metabolome Database (HMDB) is a freely available electronic database containing detailed information about small molecule metabolites found in the human body. | |
| Explanation | HMDB is offered to the public as a freely available resource. Use and re-distribution of the data, in whole or in part, for commercial purposes requires explicit permission of the authors and explicit acknowledgment of the source material (HMDB) and the original publication (see the HMDB citing page). We ask that users who download significant portions of the database cite the HMDB paper in any resulting publications. | |
Synthesis routes and methods
Procedure details







Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.













