科比替尼
描述
科比替尼,商品名为Cotellic,是一种抗癌药物,主要与维莫非尼联用治疗黑色素瘤。 它是一种选择性抑制剂,作用于丝裂原活化蛋白激酶激酶1 (MEK1) 和丝裂原活化蛋白激酶激酶2 (MEK2),它们是丝裂原活化蛋白激酶/细胞外信号调节激酶 (MAPK/ERK) 途径的一部分 。 这条途径在各种癌症中经常过度激活,这使得科比替尼成为一种有价值的治疗剂 .
科学研究应用
Indications
Cobimetinib is primarily indicated for:
- Melanoma : Used in combination with vemurafenib for patients with unresectable or metastatic melanoma harboring BRAF V600E or V600K mutations.
- Colorectal Cancer : Investigated in clinical trials for patients with BRAF-mutated colorectal cancer.
Melanoma Treatment
The pivotal study for cobimetinib's approval was the coBRIM trial , a Phase III randomized study that assessed the combination of cobimetinib and vemurafenib versus vemurafenib alone. Key findings include:
- Overall Survival (OS) : The median OS was 22.5 months for the combination therapy compared to 17.4 months for vemurafenib alone, with 5-year OS rates of 31% versus 26%, respectively .
- Progression-Free Survival (PFS) : Median PFS was significantly improved at 12.6 months compared to 7.2 months for the control group, indicating a substantial benefit from the combination therapy .
Table 1 summarizes the efficacy results from the coBRIM study:
| Measure | Cobimetinib + Vemurafenib | Vemurafenib Alone |
|---|---|---|
| Median OS | 22.5 months | 17.4 months |
| 5-Year OS Rate | 31% | 26% |
| Median PFS | 12.6 months | 7.2 months |
| 5-Year PFS Rate | 14% | 10% |
Colorectal Cancer
Cobimetinib has also shown promise in treating colorectal cancer with BRAF mutations. In a cohort study, patients receiving cobimetinib in combination with vemurafenib demonstrated improved outcomes compared to historical controls . However, further studies are necessary to establish definitive efficacy.
Safety Profile
The safety profile of cobimetinib has been consistent across studies. Common adverse events include:
- Fatigue
- Diarrhea
- Rash
- Elevated liver enzymes
In the coBRIM study, these adverse events were manageable, and no new safety signals were identified over extended follow-up periods .
Case Studies and Research Insights
- Long-Term Outcomes : Extended follow-up from the coBRIM study confirmed that patients achieving a complete response had significantly better survival outcomes, emphasizing the importance of early treatment responses .
- Combination Therapies : Ongoing research is exploring cobimetinib's effectiveness in combination with other agents targeting different pathways, such as RMC-4630 (a SHP2 inhibitor) and KRAS inhibitors . Early results indicate potential synergistic effects that may enhance treatment efficacy.
- Quality of Life : Studies have indicated that patients receiving cobimetinib plus vemurafenib report improved health-related quality of life metrics compared to those receiving monotherapy, suggesting that combination therapy may not only extend survival but also enhance patient well-being .
作用机制
生化分析
Biochemical Properties
Cobimetinib plays a crucial role in biochemical reactions by inhibiting the activity of mitogen-activated protein kinase kinase 1 (MEK1) and mitogen-activated protein kinase kinase 2 (MEK2). These enzymes are upstream regulators of the extracellular signal-regulated kinase (ERK) pathway, which promotes cellular proliferation . By inhibiting MEK1 and MEK2, cobimetinib effectively reduces the phosphorylation and activation of ERK1 and ERK2, leading to decreased cellular proliferation . Cobimetinib interacts with these enzymes through reversible binding, which allows it to inhibit their activity without permanently altering their structure .
Cellular Effects
Cobimetinib has significant effects on various types of cells and cellular processes. It primarily influences cell function by inhibiting the MEK/ERK signaling pathway, which is crucial for cell proliferation and survival . In cancer cells, cobimetinib induces apoptosis and inhibits cell growth by reducing the phosphorylation of ERK1 and ERK2 . This inhibition leads to decreased expression of genes involved in cell cycle progression and survival, ultimately resulting in reduced cellular proliferation and increased cell death . Additionally, cobimetinib has been shown to induce immunogenic cell death in certain cancer cell lines, further enhancing its anti-tumor effects .
Molecular Mechanism
The molecular mechanism of cobimetinib involves its selective inhibition of MEK1 and MEK2, which are key components of the RAS/RAF/MEK/ERK signaling pathway . Cobimetinib binds to the allosteric site of MEK1 and MEK2, preventing their activation and subsequent phosphorylation of ERK1 and ERK2 . This inhibition disrupts the downstream signaling cascade, leading to decreased cellular proliferation and increased apoptosis . Cobimetinib’s ability to maintain its inhibitory effect even when MEK is already phosphorylated further enhances its efficacy in targeting cancer cells .
Temporal Effects in Laboratory Settings
Dosage Effects in Animal Models
The effects of cobimetinib vary with different dosages in animal models. At lower doses, cobimetinib effectively inhibits tumor growth and induces apoptosis without causing significant toxicity . At higher doses, cobimetinib can cause adverse effects, including gastrointestinal toxicity, hepatotoxicity, and cardiotoxicity . These toxic effects highlight the importance of optimizing the dosage of cobimetinib to achieve maximum therapeutic benefit while minimizing adverse effects .
Metabolic Pathways
Cobimetinib is primarily metabolized through the cytochrome P450 3A4 (CYP3A4) pathway . This metabolic pathway involves the oxidation of cobimetinib, leading to the formation of various metabolites that are subsequently excreted in the feces and urine . The metabolism of cobimetinib can be influenced by other drugs that inhibit or induce CYP3A4, potentially affecting its efficacy and safety . Additionally, cobimetinib’s interaction with other enzymes and cofactors involved in its metabolism can impact its pharmacokinetics and overall therapeutic profile .
Transport and Distribution
Cobimetinib is transported and distributed within cells and tissues through various mechanisms. It is highly protein-bound, with approximately 95% of the drug bound to plasma proteins . This high protein binding affects its distribution and bioavailability, as only the unbound fraction of cobimetinib is pharmacologically active . Cobimetinib is also subject to active transport by efflux transporters, such as P-glycoprotein, which can influence its intracellular concentration and distribution . These transport mechanisms play a crucial role in determining the localization and accumulation of cobimetinib within different tissues and cells .
Subcellular Localization
The subcellular localization of cobimetinib is primarily determined by its interaction with specific targeting signals and post-translational modifications. Cobimetinib is known to localize to the cytoplasm, where it exerts its inhibitory effects on MEK1 and MEK2 . The presence of specific targeting signals, such as nuclear localization signals, can also influence the subcellular distribution of cobimetinib, directing it to specific compartments or organelles . These localization patterns are critical for cobimetinib’s activity and function, as they determine its ability to effectively inhibit the MEK/ERK signaling pathway and exert its anti-tumor effects .
准备方法
化学反应分析
科比替尼经历各种化学反应,包括:
氧化: 科比替尼主要通过细胞色素P450 3A4 (CYP3A4) 氧化代谢.
还原: 还原反应是其合成路线的一部分.
取代: 合成涉及取代反应,以引入官能团
这些反应中常用的试剂和条件包括用于 Boc 保护的叔丁氧羰基氯和用于还原步骤的各种还原剂 。 这些反应形成的主要产物是导致最终科比替尼分子的中间体 .
相似化合物的比较
科比替尼是用于癌症治疗的几种 MEK 抑制剂之一。类似化合物包括:
比尼美替尼: 与其他药物联合使用,用于治疗黑色素瘤和其他癌症.
色美替尼: 用于治疗神经纤维瘤病1型和其他癌症.
科比替尼的独特之处在于它与维莫非尼联用,维莫非尼靶向 MAPK/ERK 途径中的另一种激酶,从而产生协同效应,并改善治疗效果 .
生物活性
Cobimetinib (Cotellic™) is a selective, allosteric inhibitor of mitogen-activated protein kinase kinase (MEK), primarily used in the treatment of advanced melanoma, particularly in combination with the BRAF inhibitor vemurafenib. This article explores the biological activity of cobimetinib, its mechanisms of action, clinical efficacy, and safety profile, supported by relevant data tables and research findings.
Cobimetinib specifically inhibits MEK1 and MEK2, which are critical components of the MAPK signaling pathway. This pathway is often dysregulated in various cancers due to mutations in upstream components such as BRAF. By inhibiting MEK, cobimetinib disrupts downstream ERK signaling, leading to reduced cancer cell proliferation and survival.
- Selectivity : Cobimetinib shows a 100-fold greater potency for phosphorylated MEK1 compared to MEK2, which is crucial for its therapeutic efficacy .
Clinical Efficacy
Cobimetinib has been evaluated in several clinical trials, particularly for patients with BRAF V600E mutations. The following table summarizes key findings from major studies:
Safety Profile
While cobimetinib has demonstrated significant antitumor activity, it is associated with various adverse effects. Common side effects include:
- Diarrhea
- Photosensitivity
- Nausea and vomiting
- Severe skin rash
- Liver toxicity
Serious side effects can also occur, including heart muscle damage and retinal detachment .
Case Studies
-
Case Study on Advanced Melanoma :
A patient treated with cobimetinib and vemurafenib exhibited a dramatic reduction in tumor size after three months of therapy, with a notable improvement in quality of life. However, they experienced severe diarrhea requiring dose adjustment. -
Combination Therapy :
In another case involving a patient with SCCHN treated with cobimetinib plus atezolizumab, moderate antitumor activity was observed; however, the patient developed immune-related adverse effects that necessitated corticosteroid treatment .
Research Findings
Recent studies have further elucidated the biological activity of cobimetinib:
- Inhibition of Platelet Function : Research indicates that while cobimetinib effectively inhibits MEK activity in platelets, its impact on platelet function is less pronounced than expected at therapeutic concentrations .
- Comparative Studies : A meta-analysis highlighted that the combination of cobimetinib with other agents might enhance efficacy compared to monotherapy approaches, particularly in resistant melanoma cases .
属性
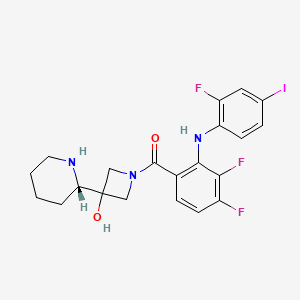
IUPAC Name |
[3,4-difluoro-2-(2-fluoro-4-iodoanilino)phenyl]-[3-hydroxy-3-[(2S)-piperidin-2-yl]azetidin-1-yl]methanone | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C21H21F3IN3O2/c22-14-6-5-13(19(18(14)24)27-16-7-4-12(25)9-15(16)23)20(29)28-10-21(30,11-28)17-3-1-2-8-26-17/h4-7,9,17,26-27,30H,1-3,8,10-11H2/t17-/m0/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
BSMCAPRUBJMWDF-KRWDZBQOSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1CCNC(C1)C2(CN(C2)C(=O)C3=C(C(=C(C=C3)F)F)NC4=C(C=C(C=C4)I)F)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
C1CCN[C@@H](C1)C2(CN(C2)C(=O)C3=C(C(=C(C=C3)F)F)NC4=C(C=C(C=C4)I)F)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C21H21F3IN3O2 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID60239435 | |
| Record name | Cobimetinib | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID60239435 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
531.3 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Mechanism of Action |
Cobimetinib is a reversible inhibitor of mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase 1 (MEK1) and MEK2. MEK proteins are upstream regulators of the extracellular signal-related kinase (ERK) pathway, which promotes cellular proliferation. BRAF V600E and K mutations result in constitutive activation of the BRAF pathway which includes MEK1 and MEK2. In mice implanted with tumor cell lines expressing BRAF V600E, cobimetinib inhibited tumor cell growth. Cobimetinib and vemurafenib target two different kinases in the RAS/RAF/MEK/ERK pathway. Compared to either drug alone, coadministration of cobimetinib and vemurafenib resulted in increased apoptosis in vitro and reduced tumor growth in mouse implantation models of tumor cell lines harboring BRAF V600E mutations. Cobimetinib also prevented vemurafenib-mediated growth enhancement of a wild-type BRAF tumor cell line in an in vivo mouse implantation model. | |
| Record name | Cobimetinib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB05239 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
CAS No. |
934660-93-2 | |
| Record name | Cobimetinib | |
| Source | CAS Common Chemistry | |
| URL | https://commonchemistry.cas.org/detail?cas_rn=934660-93-2 | |
| Description | CAS Common Chemistry is an open community resource for accessing chemical information. Nearly 500,000 chemical substances from CAS REGISTRY cover areas of community interest, including common and frequently regulated chemicals, and those relevant to high school and undergraduate chemistry classes. This chemical information, curated by our expert scientists, is provided in alignment with our mission as a division of the American Chemical Society. | |
| Explanation | The data from CAS Common Chemistry is provided under a CC-BY-NC 4.0 license, unless otherwise stated. | |
| Record name | Cobimetinib [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0934660932 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Cobimetinib | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB05239 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | Cobimetinib | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID60239435 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | COBIMETINIB | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/ER29L26N1X | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。
















