Padsevonil
Overview
Description
Padsevonil is a novel antiepileptic drug candidate developed to address the unmet needs of patients with treatment-resistant epilepsy. It is a first-in-class compound that interacts with two therapeutic targets: synaptic vesicle protein 2 and gamma-aminobutyric acid A receptors . This dual mechanism of action allows this compound to exert both presynaptic and postsynaptic effects, making it a promising option for managing epilepsy .
Preparation Methods
Padsevonil was synthesized through a medicinal chemistry program aimed at designing compounds with high affinity for synaptic vesicle 2 proteins and low-to-moderate affinity for the benzodiazepine binding site on gamma-aminobutyric acid A receptors . The specific synthetic routes and reaction conditions for this compound have not been disclosed in the available literature. it is known that the compound was developed through rational design and optimization of its pharmacological profile .
Chemical Reactions Analysis
Padsevonil undergoes various chemical reactions, including binding to synaptic vesicle 2 proteins and gamma-aminobutyric acid A receptors . The compound exhibits high affinity for synaptic vesicle 2A, 2B, and 2C isoforms, with slower binding kinetics compared to other antiepileptic drugs such as levetiracetam and brivaracetam . This compound also displays low to moderate affinity for the benzodiazepine binding site on gamma-aminobutyric acid A receptors, indicating partial agonist properties . The major products formed from these reactions include the bound complexes of this compound with its target proteins .
Scientific Research Applications
Phase IIa Trials
The efficacy of padsevonil has been evaluated in multiple clinical trials, notably the Phase IIa trial which focused on patients with treatment-resistant focal epilepsy. Key findings from this trial include:
- Participants : 55 patients were randomized, with 50 completing the trial.
- Results :
Phase IIb and III Trials
Subsequent trials (EP0091 and EP0092) further assessed this compound's safety and efficacy:
- Design : Randomized, double-blind, placebo-controlled trials involving patients experiencing focal seizures despite treatment with multiple antiepileptic drugs.
- Outcomes : Although primary outcomes did not reach statistical significance in all dose groups, this compound was generally well tolerated with a favorable safety profile .
Safety Profile
This compound has demonstrated a safety profile consistent with earlier studies:
- Adverse Events : Commonly reported treatment-emergent adverse events included somnolence (45.5%), dizziness (43.6%), and headache (25.5%). Importantly, only one patient discontinued due to these events .
- Tolerability : Overall, this compound was well tolerated among participants across various trials, indicating its potential as a viable option for patients with refractory epilepsy .
Comparative Efficacy
To contextualize this compound's efficacy, it is essential to compare it against existing antiepileptic medications:
| Drug | Mechanism of Action | Efficacy in Treatment-Resistant Epilepsy |
|---|---|---|
| This compound | SV2A binding + GABA A receptor modulation | Significant reduction in seizure frequency |
| Levetiracetam | SV2A binding | Moderate efficacy |
| Brivaracetam | SV2A binding | Moderate efficacy |
Case Studies
Several case studies have documented the real-world application of this compound:
- Case Study A : A patient with a history of multiple drug-resistant seizures experienced a significant reduction in seizure frequency after initiating treatment with this compound as an adjunct therapy.
- Case Study B : In another instance, a patient reported improved quality of life and reduced seizure episodes following the introduction of this compound alongside their existing regimen.
These cases underscore the potential benefits of this compound for individuals who have not responded adequately to traditional treatments.
Mechanism of Action
Padsevonil exerts its effects by binding to synaptic vesicle protein 2 and the benzodiazepine site on gamma-aminobutyric acid A receptors . This dual mechanism of action allows this compound to modulate both presynaptic and postsynaptic activity, resulting in enhanced seizure control . By targeting synaptic vesicle protein 2, this compound inhibits neurotransmitter release, while its interaction with gamma-aminobutyric acid A receptors enhances inhibitory neurotransmission . This combination of actions contributes to its robust efficacy in various seizure and epilepsy models .
Comparison with Similar Compounds
Padsevonil is unique among antiepileptic drugs due to its dual-target profile, which allows it to interact with both synaptic vesicle protein 2 and gamma-aminobutyric acid A receptors . Similar compounds include levetiracetam and brivaracetam, which are selective synaptic vesicle protein 2A ligands . this compound’s ability to bind to all three synaptic vesicle protein 2 isoforms and its partial agonist properties at the benzodiazepine site on gamma-aminobutyric acid A receptors set it apart from these other drugs . This unique mechanism of action provides this compound with enhanced antiseizure properties compared to the combination of compounds targeting synaptic vesicle protein 2A and the benzodiazepine site .
Biological Activity
Padsevonil, also known as UCB-0942, is a novel antiepileptic drug candidate developed by UCB Pharma. It represents a first-in-class compound targeting both synaptic vesicle protein 2A (SV2A) and GABA receptors. This dual mechanism of action is designed to enhance antiseizure efficacy while minimizing the side effects commonly associated with traditional antiepileptic drugs.
Target Interactions:
- Synaptic Vesicle Protein 2A (SV2A): this compound binds with high affinity to SV2A, which is crucial for neurotransmitter release. This interaction is believed to correlate with its antiseizure potency.
- GABA Receptors: It also interacts with GABA receptors at the benzodiazepine site, where it acts as a partial agonist. This provides therapeutic effects without inducing significant sedation or tolerance, which are common issues with full agonists.
Clinical Trials and Efficacy
This compound has undergone several clinical trials, notably a randomized Phase IIa trial focusing on treatment-resistant focal epilepsy. The key findings from this trial are summarized below:
| Parameter | This compound Group (n=24) | Placebo Group (n=26) | P-value |
|---|---|---|---|
| ≥75% Seizure Frequency Reduction | 30.8% | 11.1% | 0.067 |
| Median Weekly Seizure Frequency Reduction | 55.2% | 12.5% | 0.026 |
| Treatment-Emergent Adverse Events | 90.9% reported | 63.0% reported | - |
| Common Adverse Events | Somnolence (45.5%), Dizziness (43.6%), Headache (25.5%) | - | - |
The trial included adults experiencing at least four focal seizures per week and who had failed to respond to multiple antiepileptic drugs. After a three-week inpatient period, the results indicated a statistically significant reduction in seizure frequency among those treated with this compound compared to placebo, highlighting its potential efficacy in a challenging patient population .
Safety Profile
This compound demonstrated a favorable safety profile during the trials:
- The most common treatment-emergent adverse events included somnolence, dizziness, and headache.
- Notably, only one patient discontinued treatment due to adverse events, suggesting that this compound may be better tolerated than many existing therapies.
Translational Research Insights
Two PET imaging studies conducted in healthy volunteers helped identify optimal target occupancy levels for this compound:
Q & A
Basic Research Questions
Q. What distinguishes Padsevonil's mechanism of action from other SV2A-targeting antiepileptic drugs (AEDs) like levetiracetam?
this compound uniquely binds to all three synaptic vesicle 2 (SV2) isoforms (SV2A, SV2B, SV2C) with high affinity (pKi: SV2A = 8.5, SV2B = 7.9, SV2C = 8.5), unlike levetiracetam and brivaracetam, which selectively target SV2A. Its dissociation kinetics from SV2A are markedly slower (t1/2 >30 minutes vs. <0.5 minutes for levetiracetam/brivaracetam), suggesting prolonged target engagement. Additionally, this compound acts as a low-affinity partial agonist (40% efficacy vs. zolpidem) at GABAA receptors, combining presynaptic (SV2) and postsynaptic (GABAergic) modulation .
Q. Which preclinical models are most relevant for evaluating this compound's efficacy in drug-resistant epilepsy?
this compound demonstrated robust efficacy in the 6 Hz focal seizure model (mice) and amygdala kindling model (rodents), which predict activity against focal to bilateral tonic-clonic seizures. It also showed dose-dependent protection in chronic models like intrahippocampal kainate (mesial temporal lobe epilepsy) and Genetic Absence Epilepsy Rats from Strasbourg (GAERS). These models are prioritized due to their translational relevance to drug-resistant epilepsy .
Q. How does this compound's dual-target profile influence its therapeutic index in preclinical studies?
In rodent models, this compound exhibited a high therapeutic index, with SV2A occupancy at low doses (ED50 = 0.2 mg/kg) and GABAA receptor engagement at higher doses (ED50 = 36 mg/kg). This separation likely contributes to its favorable safety profile, avoiding excessive GABAergic side effects (e.g., sedation) at therapeutic doses .
Advanced Research Questions
Q. What methodological challenges arise when quantifying this compound's target engagement in vivo?
PET studies using SV2A radioligands (e.g., [11C]UCB-J) must account for this compound’s slow dissociation kinetics. The "Single-step" solution for occupancy calculations may underestimate binding compared to the "Numerical" method, particularly for rapid-acting drugs. Future studies should validate these models for this compound’s unique pharmacokinetic profile .
Q. How can researchers reconcile discrepancies between this compound's preclinical efficacy and mixed clinical trial outcomes?
While this compound showed superior efficacy in preclinical models (e.g., 6 Hz and amygdala kindling), phase IIb trials failed to meet primary endpoints. Potential factors include:
- Patient heterogeneity : Enrolled populations with diverse resistance mechanisms.
- Dosing limitations : Clinical doses may not achieve sufficient SV2B/C or GABAA receptor occupancy.
- Trial design : Overly optimistic endpoints (e.g., ≥75% seizure reduction) vs. preclinical benchmarks .
Q. Does this compound's multi-target action provide synergistic benefits compared to combining SV2A ligands and benzodiazepines?
In the 6 Hz model, this compound outperformed combinations of diazepam + levetiracetam/brivaracetam, suggesting its dual SV2 isoform engagement and partial GABAA agonism confer unique synergies. This contrasts with full GABAA agonists, which risk tolerance and receptor desensitization .
Q. What experimental strategies are critical for optimizing this compound's pharmacokinetic/pharmacodynamic (PK/PD) modeling?
- Microsampling techniques : Dried blood spots (DBS) or volumetric absorptive microsampling (VAMS) enable frequent sampling without compromising animal welfare.
- Cerebrospinal fluid (CSF) analysis : Directly correlates with brain exposure.
- Allometric scaling : Accounts for interspecies differences in metabolic clearance .
Q. Key Methodological Recommendations
- Binding Assays : Use recombinant SV2 isoforms and temperature-controlled (37°C) radioligand displacement to capture this compound’s slow kinetics .
- In Vivo Models : Prioritize chronic epilepsy models (e.g., intrahippocampal kainate) over acute seizure tests (e.g., pentylenetetrazol) to reflect drug-resistant mechanisms .
- Clinical Translation : Incorporate patient-centric sampling and PK/PD modeling to bridge preclinical and clinical dosing .
Properties
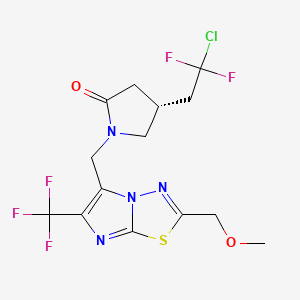
IUPAC Name |
(4R)-4-(2-chloro-2,2-difluoroethyl)-1-[[2-(methoxymethyl)-6-(trifluoromethyl)imidazo[2,1-b][1,3,4]thiadiazol-5-yl]methyl]pyrrolidin-2-one | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C14H14ClF5N4O2S/c1-26-6-9-22-24-8(11(14(18,19)20)21-12(24)27-9)5-23-4-7(2-10(23)25)3-13(15,16)17/h7H,2-6H2,1H3/t7-/m1/s1 | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
DCXFIOLWWRXEQH-SSDOTTSWSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
COCC1=NN2C(=C(N=C2S1)C(F)(F)F)CN3CC(CC3=O)CC(F)(F)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Isomeric SMILES |
COCC1=NN2C(=C(N=C2S1)C(F)(F)F)CN3C[C@H](CC3=O)CC(F)(F)Cl | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C14H14ClF5N4O2S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Weight |
432.8 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
1294000-61-5 | |
| Record name | Padsevonil [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=1294000615 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Padsevonil | |
| Source | DrugBank | |
| URL | https://www.drugbank.ca/drugs/DB14977 | |
| Description | The DrugBank database is a unique bioinformatics and cheminformatics resource that combines detailed drug (i.e. chemical, pharmacological and pharmaceutical) data with comprehensive drug target (i.e. sequence, structure, and pathway) information. | |
| Explanation | Creative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode) | |
| Record name | PADSEVONIL | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/0R1HN52K0N | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Synthesis routes and methods
Procedure details











Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.













