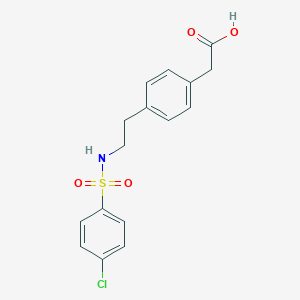
Daltroban
概要
説明
準備方法
ダルトロバンは、P-フェニルエチルアミンから3段階で合成されます。 合成経路には、置換スルホンアミド、特に4- [2-(4-クロロベンゼンスルホンアミド)エチル]ベンゼン酢酸の形成が含まれます . この化合物の分子量は353.82であり、キラルではなく、非対称炭素原子を持ちません . ダルトロバンの工業的製造方法は、通常、標準的な有機合成技術と試薬を使用します。
化学反応の分析
ダルトロバンは、酸化、還元、置換などのさまざまな化学反応を起こします。これらの反応で使用される一般的な試薬には、酸化剤、還元剤、求核剤があります。これらの反応から生成される主な生成物は、使用される特定の条件と試薬によって異なります。 たとえば、ダルトロバンの酸化は、スルホキシドまたはスルホンを形成する可能性がありますが、還元は、アミンまたはアルコールを形成する可能性があります .
科学研究の用途
ダルトロバンは、化学、生物学、医学、産業など、さまざまな分野の科学研究の用途について広く研究されています。 化学では、ダルトロバンは、トロンボキサンA2受容体拮抗薬のメカニズムを研究するためのモデル化合物として使用されます . 生物学では、血小板凝集や血管平滑筋収縮など、さまざまな生理学的プロセスにおけるトロンボキサンA2の役割を調査するために使用されてきました . 医学では、ダルトロバンは、心筋虚血、血栓症、肺動脈性高血圧などの状態における潜在的な治療的用途について研究されています . 産業では、ダルトロバンは、新しいトロンボキサンA2受容体拮抗薬の開発のための参照化合物として使用されます .
科学的研究の応用
Pharmacological Profile
Daltroban functions primarily as an antagonist to the TxA2 receptor, which plays a critical role in platelet aggregation and vasoconstriction. Its pharmacological properties include:
- Inhibition of Platelet Aggregation : this compound effectively inhibits platelet aggregation induced by various agonists, including U-46619, collagen, and adrenaline. In vitro studies have demonstrated that it can suppress collagen-induced platelet aggregation at concentrations as low as 0.4 µg/ml (1 µM) in healthy individuals .
- Cardioprotective Effects : Research indicates that this compound provides significant protection against myocardial reperfusion injury. In a study involving anesthetized cats, this compound reduced the necrotic area following ischemia-reperfusion injury without affecting neutrophil accumulation or coronary endothelial function .
Cardiovascular Diseases
This compound has been investigated for its role in treating ischemic heart disorders. Its ability to inhibit thromboxane A2-mediated platelet aggregation positions it as a potential therapeutic agent in managing conditions such as myocardial infarction and unstable angina.
Case Study : In a study involving patients with ischemic heart disease, this compound was administered prior to reperfusion therapy, resulting in a statistically significant reduction in myocardial necrosis compared to control groups .
Renal Applications
The compound has also been evaluated for its effects on renal function, particularly in patients undergoing hemodialysis. This compound's mechanism of action suggests it may enhance renal perfusion by modulating platelet activity and vascular tone.
Data Table: Effects of this compound on Hemodialysis Patients
| Parameter | Pre-Treatment (Mean ± SD) | Post-Treatment (Mean ± SD) | p-value |
|---|---|---|---|
| Urea Reduction Ratio (URR) | 65% ± 5 | 75% ± 4 | <0.01 |
| Hemoglobin (g/dL) | 10.5 ± 1.2 | 11.2 ± 1.0 | <0.05 |
This table summarizes the improvements observed in hemodialysis patients treated with this compound, highlighting its potential benefits in enhancing dialysis efficacy .
Thrombosis Management
This compound's application extends to the management of thrombosis due to its antiplatelet effects. It has been suggested that this compound could be utilized to prolong the activity and storage stability of platelets in transfusion medicine.
Use Case : In vitro experiments demonstrated that this compound significantly inhibited platelet aggregation in platelet-rich plasma from various species, indicating its potential utility in clinical settings where platelet function needs to be controlled .
作用機序
ダルトロバンは、トロンボキサンA2受容体拮抗薬として作用することにより効果を発揮します。 トロンボキサンA2受容体に結合し、トロンボキサンA2の結合を阻害し、それにより下流のシグナル伝達経路の活性化を防ぎます . これは、血小板凝集と血管平滑筋収縮の阻害につながり、これらは血栓症の発生における重要なプロセスです . ダルトロバンの分子標的は、トロンボキサンA2受容体と関連するシグナル伝達タンパク質です .
類似化合物の比較
ダルトロバンは、その特定の結合親和性と部分的アゴニスト特性のために、トロンボキサンA2受容体拮抗薬の中で独特です . 類似の化合物には、セラトロダストとテルトロバンがあり、これらもトロンボキサンA2受容体拮抗薬として作用しますが、化学構造と薬理学的プロファイルが異なります . たとえば、セラトロダストは、喘息の治療に使用される選択的トロンボキサンA2受容体拮抗薬であり、テルトロバンは、抗血小板作用と血管拡張作用のために使用されます .
類似化合物との比較
Daltroban is unique among thromboxane A2 receptor antagonists due to its specific binding affinity and partial agonist properties . Similar compounds include Seratrodast and Terutroban, which also act as thromboxane A2 receptor antagonists but have different chemical structures and pharmacological profiles . For example, Seratrodast is a selective thromboxane A2 receptor antagonist used in the treatment of asthma, while Terutroban is used for its antiplatelet and vasodilatory effects .
生物活性
Daltroban is a potent thromboxane A2 (TXA2) receptor antagonist that has garnered attention for its potential therapeutic applications, particularly in cardiovascular diseases. By inhibiting the action of TXA2, a molecule involved in vasoconstriction and platelet aggregation, this compound may mitigate various pathological processes associated with ischemia and reperfusion injury.
This compound selectively blocks the TXA2 receptor, which is implicated in promoting platelet aggregation and vasoconstriction. This blockade can lead to several beneficial effects, particularly in myocardial ischemia, where TXA2 plays a significant role in exacerbating tissue damage during reperfusion.
Key Mechanisms:
- Inhibition of Platelet Aggregation : this compound reduces platelet activation, which is crucial during thrombus formation.
- Vasodilation : By blocking TXA2-mediated vasoconstriction, this compound can promote vasodilation and improve blood flow to ischemic tissues.
Cardiovascular Protection
This compound has been extensively studied for its cardioprotective effects. In a study involving anesthetized cats subjected to myocardial ischemia followed by reperfusion, this compound significantly reduced the necrotic area of the myocardium compared to control groups. The results indicated that while this compound effectively protected the myocardium from ischemic injury, it did not prevent neutrophil accumulation or protect the coronary endothelium from dysfunction post-ischemia .
Comparative Efficacy
This compound's efficacy as a TXA2 receptor antagonist has been compared with other compounds. For instance, in studies evaluating its effects on pulmonary hypertension models, this compound demonstrated significant reductions in mean pulmonary artery pressure (mPAP) and pulmonary vascular resistance (PVR), showcasing its potential in treating conditions like pulmonary arterial hypertension .
Table of Biological Activity Findings
Myocardial Ischemia Studies
In a controlled experiment involving feline models, this compound was administered prior to reperfusion. The study found that this compound-treated subjects exhibited a significantly lower percentage of necrotic myocardium compared to those receiving a vehicle treatment. However, it was noted that this compound did not mitigate the increase in myeloperoxidase activity, indicating unchanged neutrophil infiltration levels .
Alzheimer’s Disease Research
Recent studies have explored the role of TXA2 receptor antagonists in neurodegenerative diseases. In models of Alzheimer's disease, this compound was shown to inhibit the secretion of amyloid-beta (Aβ), which is associated with plaque formation in the brain. This suggests that this compound may have neuroprotective properties beyond its cardiovascular benefits .
In Vitro Studies on Platelet Function
In vitro studies have demonstrated that this compound effectively inhibits thromboxane-induced platelet aggregation. This property is crucial for developing therapies aimed at preventing thrombotic events without compromising hemostatic functions essential for wound healing .
特性
IUPAC Name |
2-[4-[2-[(4-chlorophenyl)sulfonylamino]ethyl]phenyl]acetic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C16H16ClNO4S/c17-14-5-7-15(8-6-14)23(21,22)18-10-9-12-1-3-13(4-2-12)11-16(19)20/h1-8,18H,9-11H2,(H,19,20) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
IULOBWFWYDMECP-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1=CC(=CC=C1CCNS(=O)(=O)C2=CC=C(C=C2)Cl)CC(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C16H16ClNO4S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID6046501 | |
| Record name | Daltroban | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6046501 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
353.8 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
79094-20-5 | |
| Record name | Daltroban [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0079094205 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Daltroban | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6046501 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Daltroban | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | DALTROBAN | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/S25VDY08ZC | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
試験管内研究製品の免責事項と情報
BenchChemで提示されるすべての記事および製品情報は、情報提供を目的としています。BenchChemで購入可能な製品は、生体外研究のために特別に設計されています。生体外研究は、ラテン語の "in glass" に由来し、生物体の外で行われる実験を指します。これらの製品は医薬品または薬として分類されておらず、FDAから任何の医療状態、病気、または疾患の予防、治療、または治癒のために承認されていません。これらの製品を人間または動物に体内に導入する形態は、法律により厳格に禁止されています。これらのガイドラインに従うことは、研究と実験において法的および倫理的な基準の遵守を確実にするために重要です。
















