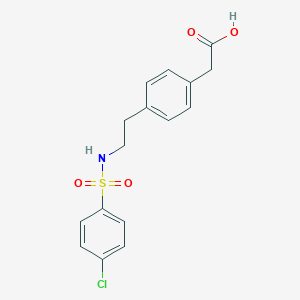
Daltroban
描述
准备方法
达特罗班由对苯乙胺通过三步合成。 合成路线涉及形成取代的磺酰胺,具体为4-[2-(4-氯苯磺酰胺基)乙基]苯乙酸 . 该化合物分子量为353.82,且为非手性化合物,意味着其没有不对称碳原子 . 达特罗班的工业生产方法通常涉及使用标准的有机合成技术和试剂。
化学反应分析
达特罗班会发生各种化学反应,包括氧化、还原和取代。这些反应中常用的试剂包括氧化剂、还原剂和亲核试剂。这些反应形成的主要产物取决于所使用的具体条件和试剂。 例如,达特罗班的氧化会导致形成亚砜或砜,而还原会导致形成胺或醇 .
科学研究应用
Pharmacological Profile
Daltroban functions primarily as an antagonist to the TxA2 receptor, which plays a critical role in platelet aggregation and vasoconstriction. Its pharmacological properties include:
- Inhibition of Platelet Aggregation : this compound effectively inhibits platelet aggregation induced by various agonists, including U-46619, collagen, and adrenaline. In vitro studies have demonstrated that it can suppress collagen-induced platelet aggregation at concentrations as low as 0.4 µg/ml (1 µM) in healthy individuals .
- Cardioprotective Effects : Research indicates that this compound provides significant protection against myocardial reperfusion injury. In a study involving anesthetized cats, this compound reduced the necrotic area following ischemia-reperfusion injury without affecting neutrophil accumulation or coronary endothelial function .
Cardiovascular Diseases
This compound has been investigated for its role in treating ischemic heart disorders. Its ability to inhibit thromboxane A2-mediated platelet aggregation positions it as a potential therapeutic agent in managing conditions such as myocardial infarction and unstable angina.
Case Study : In a study involving patients with ischemic heart disease, this compound was administered prior to reperfusion therapy, resulting in a statistically significant reduction in myocardial necrosis compared to control groups .
Renal Applications
The compound has also been evaluated for its effects on renal function, particularly in patients undergoing hemodialysis. This compound's mechanism of action suggests it may enhance renal perfusion by modulating platelet activity and vascular tone.
Data Table: Effects of this compound on Hemodialysis Patients
| Parameter | Pre-Treatment (Mean ± SD) | Post-Treatment (Mean ± SD) | p-value |
|---|---|---|---|
| Urea Reduction Ratio (URR) | 65% ± 5 | 75% ± 4 | <0.01 |
| Hemoglobin (g/dL) | 10.5 ± 1.2 | 11.2 ± 1.0 | <0.05 |
This table summarizes the improvements observed in hemodialysis patients treated with this compound, highlighting its potential benefits in enhancing dialysis efficacy .
Thrombosis Management
This compound's application extends to the management of thrombosis due to its antiplatelet effects. It has been suggested that this compound could be utilized to prolong the activity and storage stability of platelets in transfusion medicine.
Use Case : In vitro experiments demonstrated that this compound significantly inhibited platelet aggregation in platelet-rich plasma from various species, indicating its potential utility in clinical settings where platelet function needs to be controlled .
作用机制
达特罗班通过作为血栓烷A2受体拮抗剂发挥作用。 它与血栓烷A2受体结合并抑制血栓烷A2的结合,从而阻止下游信号通路的激活 . 这会导致血小板聚集和血管平滑肌收缩的抑制,这两者是血栓形成事件发生的关键过程 . 达特罗班的分子靶标包括血栓烷A2受体和相关的信号蛋白 .
相似化合物的比较
生物活性
Daltroban is a potent thromboxane A2 (TXA2) receptor antagonist that has garnered attention for its potential therapeutic applications, particularly in cardiovascular diseases. By inhibiting the action of TXA2, a molecule involved in vasoconstriction and platelet aggregation, this compound may mitigate various pathological processes associated with ischemia and reperfusion injury.
This compound selectively blocks the TXA2 receptor, which is implicated in promoting platelet aggregation and vasoconstriction. This blockade can lead to several beneficial effects, particularly in myocardial ischemia, where TXA2 plays a significant role in exacerbating tissue damage during reperfusion.
Key Mechanisms:
- Inhibition of Platelet Aggregation : this compound reduces platelet activation, which is crucial during thrombus formation.
- Vasodilation : By blocking TXA2-mediated vasoconstriction, this compound can promote vasodilation and improve blood flow to ischemic tissues.
Cardiovascular Protection
This compound has been extensively studied for its cardioprotective effects. In a study involving anesthetized cats subjected to myocardial ischemia followed by reperfusion, this compound significantly reduced the necrotic area of the myocardium compared to control groups. The results indicated that while this compound effectively protected the myocardium from ischemic injury, it did not prevent neutrophil accumulation or protect the coronary endothelium from dysfunction post-ischemia .
Comparative Efficacy
This compound's efficacy as a TXA2 receptor antagonist has been compared with other compounds. For instance, in studies evaluating its effects on pulmonary hypertension models, this compound demonstrated significant reductions in mean pulmonary artery pressure (mPAP) and pulmonary vascular resistance (PVR), showcasing its potential in treating conditions like pulmonary arterial hypertension .
Table of Biological Activity Findings
Myocardial Ischemia Studies
In a controlled experiment involving feline models, this compound was administered prior to reperfusion. The study found that this compound-treated subjects exhibited a significantly lower percentage of necrotic myocardium compared to those receiving a vehicle treatment. However, it was noted that this compound did not mitigate the increase in myeloperoxidase activity, indicating unchanged neutrophil infiltration levels .
Alzheimer’s Disease Research
Recent studies have explored the role of TXA2 receptor antagonists in neurodegenerative diseases. In models of Alzheimer's disease, this compound was shown to inhibit the secretion of amyloid-beta (Aβ), which is associated with plaque formation in the brain. This suggests that this compound may have neuroprotective properties beyond its cardiovascular benefits .
In Vitro Studies on Platelet Function
In vitro studies have demonstrated that this compound effectively inhibits thromboxane-induced platelet aggregation. This property is crucial for developing therapies aimed at preventing thrombotic events without compromising hemostatic functions essential for wound healing .
属性
IUPAC Name |
2-[4-[2-[(4-chlorophenyl)sulfonylamino]ethyl]phenyl]acetic acid | |
|---|---|---|
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI |
InChI=1S/C16H16ClNO4S/c17-14-5-7-15(8-6-14)23(21,22)18-10-9-12-1-3-13(4-2-12)11-16(19)20/h1-8,18H,9-11H2,(H,19,20) | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
InChI Key |
IULOBWFWYDMECP-UHFFFAOYSA-N | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Canonical SMILES |
C1=CC(=CC=C1CCNS(=O)(=O)C2=CC=C(C=C2)Cl)CC(=O)O | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
Molecular Formula |
C16H16ClNO4S | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
DSSTOX Substance ID |
DTXSID6046501 | |
| Record name | Daltroban | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6046501 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
Molecular Weight |
353.8 g/mol | |
| Source | PubChem | |
| URL | https://pubchem.ncbi.nlm.nih.gov | |
| Description | Data deposited in or computed by PubChem | |
CAS No. |
79094-20-5 | |
| Record name | Daltroban [USAN:INN] | |
| Source | ChemIDplus | |
| URL | https://pubchem.ncbi.nlm.nih.gov/substance/?source=chemidplus&sourceid=0079094205 | |
| Description | ChemIDplus is a free, web search system that provides access to the structure and nomenclature authority files used for the identification of chemical substances cited in National Library of Medicine (NLM) databases, including the TOXNET system. | |
| Record name | Daltroban | |
| Source | EPA DSSTox | |
| URL | https://comptox.epa.gov/dashboard/DTXSID6046501 | |
| Description | DSSTox provides a high quality public chemistry resource for supporting improved predictive toxicology. | |
| Record name | Daltroban | |
| Source | European Chemicals Agency (ECHA) | |
| URL | https://echa.europa.eu/information-on-chemicals | |
| Description | The European Chemicals Agency (ECHA) is an agency of the European Union which is the driving force among regulatory authorities in implementing the EU's groundbreaking chemicals legislation for the benefit of human health and the environment as well as for innovation and competitiveness. | |
| Explanation | Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. | |
| Record name | DALTROBAN | |
| Source | FDA Global Substance Registration System (GSRS) | |
| URL | https://gsrs.ncats.nih.gov/ginas/app/beta/substances/S25VDY08ZC | |
| Description | The FDA Global Substance Registration System (GSRS) enables the efficient and accurate exchange of information on what substances are in regulated products. Instead of relying on names, which vary across regulatory domains, countries, and regions, the GSRS knowledge base makes it possible for substances to be defined by standardized, scientific descriptions. | |
| Explanation | Unless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required. | |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
体外研究产品的免责声明和信息
请注意,BenchChem 上展示的所有文章和产品信息仅供信息参考。 BenchChem 上可购买的产品专为体外研究设计,这些研究在生物体外进行。体外研究,源自拉丁语 "in glass",涉及在受控实验室环境中使用细胞或组织进行的实验。重要的是要注意,这些产品没有被归类为药物或药品,他们没有得到 FDA 的批准,用于预防、治疗或治愈任何医疗状况、疾病或疾病。我们必须强调,将这些产品以任何形式引入人类或动物的身体都是法律严格禁止的。遵守这些指南对确保研究和实验的法律和道德标准的符合性至关重要。
















