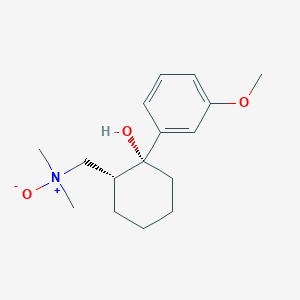
Tramadol N-Oxide
Übersicht
Beschreibung
Tramadol N-oxide (TNO) is a novel analgesic agent that is believed to exert its effects following metabolic conversion to tramadol . Tramadol itself is a widely prescribed central nervous system analgesic used for the treatment of moderate to severe pain . This compound is a prodrug, meaning it is converted into the active drug (tramadol) within the body, thereby extending its analgesic effects .
Vorbereitungsmethoden
Synthetic Routes and Reaction Conditions: The synthesis of Tramadol N-oxide involves the N-oxidation of tramadol. This can be achieved using various oxidizing agents such as hydrogen peroxide or peracids under controlled conditions . The reaction typically requires a solvent like methanol or ethanol and is carried out at room temperature to avoid decomposition of the product.
Industrial Production Methods: Industrial production of this compound follows similar synthetic routes but on a larger scale. The process involves the use of continuous flow reactors to ensure consistent product quality and yield. The reaction conditions are optimized to maximize the conversion rate and minimize by-products .
Analyse Chemischer Reaktionen
Arten von Reaktionen: Tramadol-N-oxid unterliegt verschiedenen Arten von chemischen Reaktionen, darunter:
N-Oxid-Reduktion: Diese Reaktion wandelt Tramadol-N-oxid wieder in Tramadol um, die aktive analgetische Form.
O-Demethylierung: Diese Reaktion beinhaltet die Entfernung einer Methylgruppe vom Sauerstoffatom, was zur Bildung von Desmethyltramadol führt.
N-Demethylierung: Diese Reaktion entfernt eine Methylgruppe vom Stickstoffatom und erzeugt Nortramadol.
Cyclohexylhydroxylierung: Diese Reaktion fügt dem Cyclohexylring eine Hydroxylgruppe hinzu.
Häufige Reagenzien und Bedingungen:
N-Oxid-Reduktion: Umfasst typischerweise die Verwendung von Reduktionsmitteln wie Zinkstaub oder katalytische Hydrierung.
O-Demethylierung und N-Demethylierung: Diese Reaktionen erfordern oft starke Säuren oder Basen, wie Salzsäure oder Natriumhydroxid, unter Rückflussbedingungen.
Cyclohexylhydroxylierung: Diese Reaktion wird normalerweise mit Oxidationsmitteln wie Kaliumpermanganat oder Osmiumtetroxid durchgeführt.
Hauptprodukte:
Tramadol: Das Hauptprodukt der N-Oxid-Reduktion.
Desmethyltramadol: Das Produkt der O-Demethylierung.
Nortramadol: Das Produkt der N-Demethylierung.
Hydroxylierte Tramadol-Derivate: Produkte der Cyclohexylhydroxylierung.
Wissenschaftliche Forschungsanwendungen
Tramadol-N-oxid hat verschiedene Anwendungen in der wissenschaftlichen Forschung, darunter:
Biologie: Untersucht für seine Stoffwechselwege und Wechselwirkungen mit verschiedenen Enzymen.
Medizin: Untersucht auf sein Potenzial als Prodrug, um die analgetische Wirkung von Tramadol zu verstärken.
Industrie: Wird bei der Entwicklung neuer Analgetika-Formulierungen und Medikamentenabgabesysteme eingesetzt.
5. Wirkmechanismus
Tramadol-N-oxid entfaltet seine Wirkung hauptsächlich durch seine Umwandlung in Tramadol. Tramadol hat sowohl opioid- als auch nicht-opioid-basierte Wirkmechanismen. Es wirkt als Agonist des μ-Opioidrezeptors und hemmt die Wiederaufnahme von Serotonin und Noradrenalin . Dieser duale Mechanismus trägt zu seinen analgetischen Eigenschaften bei, indem er Schmerzsignale im zentralen Nervensystem moduliert .
Ähnliche Verbindungen:
Tramadol: Die Stammverbindung von Tramadol-N-oxid mit ähnlichen analgetischen Eigenschaften.
Desmethyltramadol: Ein Metabolit von Tramadol mit reduzierter analgetischer Potenz.
Nortramadol: Ein weiterer Metabolit mit unterschiedlichen pharmakologischen Eigenschaften.
Einzigartigkeit: Tramadol-N-oxid ist einzigartig in seiner Fähigkeit, als Prodrug zu dienen, wodurch eine anhaltende Freisetzung von Tramadol erzielt und möglicherweise die Dosierungsfrequenz reduziert werden kann . Seine N-Oxid-Funktionalität bietet auch eine eindeutige chemische Reaktivität, was es zu einer wertvollen Verbindung für Forschung und Entwicklung in der pharmazeutischen Chemie macht .
Wirkmechanismus
Tramadol N-oxide exerts its effects primarily through its conversion to tramadol. Tramadol has both opioid and non-opioid mechanisms of action. It acts as an agonist of the μ-opioid receptor and inhibits the reuptake of serotonin and norepinephrine . This dual mechanism contributes to its analgesic properties by modulating pain signals in the central nervous system .
Vergleich Mit ähnlichen Verbindungen
Tramadol: The parent compound of Tramadol N-oxide, with similar analgesic properties.
Desmethyl tramadol: A metabolite of tramadol with reduced analgesic potency.
Nortramadol: Another metabolite with distinct pharmacological properties.
Uniqueness: this compound is unique in its ability to serve as a prodrug, providing a sustained release of tramadol and potentially reducing the frequency of dosing . Its N-oxide functionality also offers distinct chemical reactivity, making it a valuable compound for research and development in medicinal chemistry .
Biologische Aktivität
Tramadol N-Oxide (TNO), also known as RWJ-38705, is a metabolite of tramadol, a widely used analgesic. This compound has gained attention due to its unique pharmacological properties, which differ significantly from those of its parent drug. This article explores the biological activity of TNO, including its metabolism, analgesic effects, and potential clinical applications.
Metabolism of this compound
TNO undergoes extensive metabolism in various species, including humans. Studies have shown that TNO is metabolized primarily in the liver, with significant conversion to tramadol occurring in both rat and human hepatic S9 fractions. The metabolic pathways include:
- N-Oxide Reduction
- O-Demethylation
- N-Demethylation
- Cyclohexylhydroxylation
In vitro studies demonstrated that unchanged TNO constituted 60% of the sample in mouse hepatic fractions, while this percentage was 24% in rats and 26% in humans . The conversion of TNO to tramadol is rapid and substantial, suggesting that TNO acts as a prodrug for tramadol.
Analgesic Activity
TNO exhibits notable analgesic properties that are distinct from those of tramadol. Research indicates that TNO produces dose-related antinociceptive effects across various pain models:
- Mouse Abdominal Irritant Test : ED50 = 15.5 mg/kg
- Hot-Plate Test (48°C) : ED50 = 84.7 mg/kg
- Tail-Flick Test : ED50 = 316.4 mg/kg
- Hot-Plate Test (55°C) : ED50 = 138.2 mg/kg
The duration of action for TNO is significantly extended compared to tramadol, lasting approximately 4-5 hours . Importantly, TNO has minimal affinity for traditional opioid receptors (mu, delta, kappa), indicating a different mechanism of action than typical opioids .
Side Effects and Advantages
TNO offers several advantages over tramadol and traditional opioids:
- Reduced Side Effects : TNO is associated with fewer side effects such as respiratory depression and constipation, which are common with opioid analgesics .
- Longer Duration of Action : Patients may benefit from longer-lasting pain relief without the need for frequent dosing .
- Prodrug Characteristics : As a prodrug, TNO's conversion to tramadol allows for effective pain management while minimizing the risk of abuse associated with direct opioid administration .
Case Studies and Clinical Implications
Case studies have highlighted the potential clinical benefits of TNO:
- Chronic Pain Management : In patients requiring long-term analgesia, TNO may provide effective pain relief with a lower incidence of adverse effects compared to traditional opioids.
- Post-Surgical Pain Control : Studies suggest that TNO could be beneficial in managing postoperative pain due to its prolonged analgesic effects.
Comparative Analysis of Tramadol and this compound
The following table summarizes key differences between tramadol and its N-Oxide derivative:
| Feature | Tramadol | This compound |
|---|---|---|
| Analgesic Mechanism | Opioid receptor agonist | Prodrug; minimal opioid receptor affinity |
| Duration of Action | Shorter (2-4 hours) | Longer (4-5 hours) |
| Side Effects | Respiratory depression, constipation | Fewer side effects |
| Metabolism | Hepatic metabolism | Extensive hepatic metabolism to tramadol |
Eigenschaften
Molekularformel |
C16H25NO3 |
|---|---|
Molekulargewicht |
279.37 g/mol |
IUPAC-Name |
1-[(1R,2R)-2-hydroxy-2-(3-methoxyphenyl)cyclohexyl]-N,N-dimethylmethanamine oxide |
InChI |
InChI=1S/C16H25NO3/c1-17(2,19)12-14-7-4-5-10-16(14,18)13-8-6-9-15(11-13)20-3/h6,8-9,11,14,18H,4-5,7,10,12H2,1-3H3/t14-,16+/m1/s1 |
InChI-Schlüssel |
HBXKSXMNNGHBEA-ZBFHGGJFSA-N |
Isomerische SMILES |
C[N+](C)(C[C@H]1CCCC[C@@]1(C2=CC(=CC=C2)OC)O)[O-] |
Kanonische SMILES |
C[N+](C)(CC1CCCCC1(C2=CC(=CC=C2)OC)O)[O-] |
Synonyme |
2-((dimethylamino)methyl)-1-(3-methoxyphenyl)cyclohexanol hydrochloride RWJ 38705 tramadol N-oxide |
Herkunft des Produkts |
United States |
Synthesis routes and methods I
Procedure details





Synthesis routes and methods II
Procedure details





Haftungsausschluss und Informationen zu In-Vitro-Forschungsprodukten
Bitte beachten Sie, dass alle Artikel und Produktinformationen, die auf BenchChem präsentiert werden, ausschließlich zu Informationszwecken bestimmt sind. Die auf BenchChem zum Kauf angebotenen Produkte sind speziell für In-vitro-Studien konzipiert, die außerhalb lebender Organismen durchgeführt werden. In-vitro-Studien, abgeleitet von dem lateinischen Begriff "in Glas", beinhalten Experimente, die in kontrollierten Laborumgebungen unter Verwendung von Zellen oder Geweben durchgeführt werden. Es ist wichtig zu beachten, dass diese Produkte nicht als Arzneimittel oder Medikamente eingestuft sind und keine Zulassung der FDA für die Vorbeugung, Behandlung oder Heilung von medizinischen Zuständen, Beschwerden oder Krankheiten erhalten haben. Wir müssen betonen, dass jede Form der körperlichen Einführung dieser Produkte in Menschen oder Tiere gesetzlich strikt untersagt ist. Es ist unerlässlich, sich an diese Richtlinien zu halten, um die Einhaltung rechtlicher und ethischer Standards in Forschung und Experiment zu gewährleisten.













