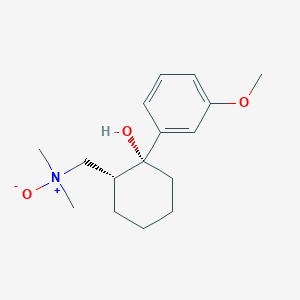
Tramadol N-Oxide
Vue d'ensemble
Description
Le Tramadol N-oxyde (TNO) est un nouvel analgésique qui exerce ses effets après conversion métabolique en tramadol . Le Tramadol lui-même est un analgésique du système nerveux central largement prescrit pour le traitement de la douleur modérée à sévère . Le Tramadol N-oxyde est un promédicament, ce qui signifie qu'il est converti en médicament actif (tramadol) dans l'organisme, prolongeant ainsi ses effets analgésiques .
Méthodes De Préparation
Voies de synthèse et conditions de réaction : La synthèse du Tramadol N-oxyde implique la N-oxydation du tramadol. Cela peut être réalisé en utilisant divers agents oxydants tels que le peroxyde d'hydrogène ou les peracides dans des conditions contrôlées . La réaction nécessite généralement un solvant comme le méthanol ou l'éthanol et est effectuée à température ambiante pour éviter la décomposition du produit.
Méthodes de production industrielle : La production industrielle du Tramadol N-oxyde suit des voies de synthèse similaires mais à plus grande échelle. Le processus implique l'utilisation de réacteurs à écoulement continu pour garantir une qualité et un rendement constants du produit. Les conditions de réaction sont optimisées pour maximiser le taux de conversion et minimiser les sous-produits .
Analyse Des Réactions Chimiques
Types de réactions : Le Tramadol N-oxyde subit plusieurs types de réactions chimiques, notamment :
Réduction du N-oxyde : Cette réaction convertit le Tramadol N-oxyde en tramadol, qui est la forme analgésique active.
O-déméthylation : Cette réaction implique l'élimination d'un groupe méthyle de l'atome d'oxygène, conduisant à la formation de désméthyltramadol.
N-déméthylation : Cette réaction élimine un groupe méthyle de l'atome d'azote, produisant du nortramadol.
Cyclohexylhydroxylation : Cette réaction ajoute un groupe hydroxyle au cycle cyclohexyle.
Réactifs et conditions communes :
Réduction du N-oxyde : Implique généralement l'utilisation d'agents réducteurs comme la poudre de zinc ou l'hydrogénation catalytique.
O-déméthylation et N-déméthylation : Ces réactions nécessitent souvent des acides ou des bases forts, tels que l'acide chlorhydrique ou l'hydroxyde de sodium, sous des conditions de reflux.
Cyclohexylhydroxylation : Cette réaction est généralement effectuée en utilisant des agents oxydants comme le permanganate de potassium ou le tétroxyde d'osmium.
Principaux produits :
Tramadol : Le produit principal de la réduction du N-oxyde.
Désméthyltramadol : Le produit de l'O-déméthylation.
Nortramadol : Le produit de la N-déméthylation.
Dérivés hydroxylés du tramadol : Produits de la cyclohexylhydroxylation.
4. Applications de recherche scientifique
Le Tramadol N-oxyde a plusieurs applications de recherche scientifique, notamment :
Chimie : Utilisé comme composé modèle pour étudier la réduction du N-oxyde et d'autres réactions connexes.
Biologie : Étudié pour ses voies métaboliques et ses interactions avec diverses enzymes.
Médecine : Exploré pour son potentiel comme promédicament pour améliorer les effets analgésiques du tramadol.
Industrie : Utilisé dans le développement de nouvelles formulations analgésiques et de systèmes d'administration de médicaments.
5. Mécanisme d'action
Le Tramadol N-oxyde exerce ses effets principalement par sa conversion en tramadol. Le tramadol a à la fois des mécanismes d'action opioïdes et non opioïdes. Il agit comme un agoniste du récepteur μ-opioïde et inhibe la recapture de la sérotonine et de la noradrénaline . Ce double mécanisme contribue à ses propriétés analgésiques en modulant les signaux de douleur dans le système nerveux central .
Composés similaires :
Tramadol : Le composé parent du Tramadol N-oxyde, avec des propriétés analgésiques similaires.
Désméthyltramadol : Un métabolite du tramadol avec une puissance analgésique réduite.
Nortramadol : Un autre métabolite avec des propriétés pharmacologiques distinctes.
Unicité : Le Tramadol N-oxyde est unique en sa capacité à servir de promédicament, fournissant une libération prolongée de tramadol et réduisant potentiellement la fréquence de l'administration . Sa fonctionnalité N-oxyde offre également une réactivité chimique distincte, ce qui en fait un composé précieux pour la recherche et le développement en chimie médicinale .
Applications De Recherche Scientifique
Tramadol N-oxide has several scientific research applications, including:
Chemistry: Used as a model compound to study N-oxide reduction and other related reactions.
Biology: Investigated for its metabolic pathways and interactions with various enzymes.
Medicine: Explored for its potential as a prodrug to enhance the analgesic effects of tramadol.
Industry: Utilized in the development of new analgesic formulations and drug delivery systems.
Mécanisme D'action
Tramadol N-oxide exerts its effects primarily through its conversion to tramadol. Tramadol has both opioid and non-opioid mechanisms of action. It acts as an agonist of the μ-opioid receptor and inhibits the reuptake of serotonin and norepinephrine . This dual mechanism contributes to its analgesic properties by modulating pain signals in the central nervous system .
Comparaison Avec Des Composés Similaires
Tramadol: The parent compound of Tramadol N-oxide, with similar analgesic properties.
Desmethyl tramadol: A metabolite of tramadol with reduced analgesic potency.
Nortramadol: Another metabolite with distinct pharmacological properties.
Uniqueness: this compound is unique in its ability to serve as a prodrug, providing a sustained release of tramadol and potentially reducing the frequency of dosing . Its N-oxide functionality also offers distinct chemical reactivity, making it a valuable compound for research and development in medicinal chemistry .
Activité Biologique
Tramadol N-Oxide (TNO), also known as RWJ-38705, is a metabolite of tramadol, a widely used analgesic. This compound has gained attention due to its unique pharmacological properties, which differ significantly from those of its parent drug. This article explores the biological activity of TNO, including its metabolism, analgesic effects, and potential clinical applications.
Metabolism of this compound
TNO undergoes extensive metabolism in various species, including humans. Studies have shown that TNO is metabolized primarily in the liver, with significant conversion to tramadol occurring in both rat and human hepatic S9 fractions. The metabolic pathways include:
- N-Oxide Reduction
- O-Demethylation
- N-Demethylation
- Cyclohexylhydroxylation
In vitro studies demonstrated that unchanged TNO constituted 60% of the sample in mouse hepatic fractions, while this percentage was 24% in rats and 26% in humans . The conversion of TNO to tramadol is rapid and substantial, suggesting that TNO acts as a prodrug for tramadol.
Analgesic Activity
TNO exhibits notable analgesic properties that are distinct from those of tramadol. Research indicates that TNO produces dose-related antinociceptive effects across various pain models:
- Mouse Abdominal Irritant Test : ED50 = 15.5 mg/kg
- Hot-Plate Test (48°C) : ED50 = 84.7 mg/kg
- Tail-Flick Test : ED50 = 316.4 mg/kg
- Hot-Plate Test (55°C) : ED50 = 138.2 mg/kg
The duration of action for TNO is significantly extended compared to tramadol, lasting approximately 4-5 hours . Importantly, TNO has minimal affinity for traditional opioid receptors (mu, delta, kappa), indicating a different mechanism of action than typical opioids .
Side Effects and Advantages
TNO offers several advantages over tramadol and traditional opioids:
- Reduced Side Effects : TNO is associated with fewer side effects such as respiratory depression and constipation, which are common with opioid analgesics .
- Longer Duration of Action : Patients may benefit from longer-lasting pain relief without the need for frequent dosing .
- Prodrug Characteristics : As a prodrug, TNO's conversion to tramadol allows for effective pain management while minimizing the risk of abuse associated with direct opioid administration .
Case Studies and Clinical Implications
Case studies have highlighted the potential clinical benefits of TNO:
- Chronic Pain Management : In patients requiring long-term analgesia, TNO may provide effective pain relief with a lower incidence of adverse effects compared to traditional opioids.
- Post-Surgical Pain Control : Studies suggest that TNO could be beneficial in managing postoperative pain due to its prolonged analgesic effects.
Comparative Analysis of Tramadol and this compound
The following table summarizes key differences between tramadol and its N-Oxide derivative:
| Feature | Tramadol | This compound |
|---|---|---|
| Analgesic Mechanism | Opioid receptor agonist | Prodrug; minimal opioid receptor affinity |
| Duration of Action | Shorter (2-4 hours) | Longer (4-5 hours) |
| Side Effects | Respiratory depression, constipation | Fewer side effects |
| Metabolism | Hepatic metabolism | Extensive hepatic metabolism to tramadol |
Propriétés
Formule moléculaire |
C16H25NO3 |
|---|---|
Poids moléculaire |
279.37 g/mol |
Nom IUPAC |
1-[(1R,2R)-2-hydroxy-2-(3-methoxyphenyl)cyclohexyl]-N,N-dimethylmethanamine oxide |
InChI |
InChI=1S/C16H25NO3/c1-17(2,19)12-14-7-4-5-10-16(14,18)13-8-6-9-15(11-13)20-3/h6,8-9,11,14,18H,4-5,7,10,12H2,1-3H3/t14-,16+/m1/s1 |
Clé InChI |
HBXKSXMNNGHBEA-ZBFHGGJFSA-N |
SMILES isomérique |
C[N+](C)(C[C@H]1CCCC[C@@]1(C2=CC(=CC=C2)OC)O)[O-] |
SMILES canonique |
C[N+](C)(CC1CCCCC1(C2=CC(=CC=C2)OC)O)[O-] |
Synonymes |
2-((dimethylamino)methyl)-1-(3-methoxyphenyl)cyclohexanol hydrochloride RWJ 38705 tramadol N-oxide |
Origine du produit |
United States |
Synthesis routes and methods I
Procedure details





Synthesis routes and methods II
Procedure details





Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.













