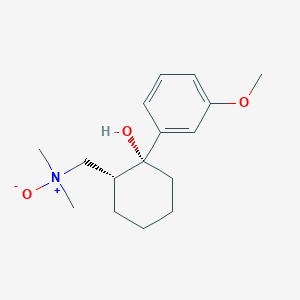
Tramadol N-Oxide
Descripción general
Descripción
El Tramadol N-óxido (TNO) es un nuevo agente analgésico que se cree que ejerce sus efectos después de la conversión metabólica a tramadol . El propio tramadol es un analgésico del sistema nervioso central ampliamente recetado para el tratamiento del dolor moderado a severo . El Tramadol N-óxido es un profármaco, lo que significa que se convierte en el fármaco activo (tramadol) dentro del cuerpo, extendiendo así sus efectos analgésicos .
Métodos De Preparación
Rutas sintéticas y condiciones de reacción: La síntesis de Tramadol N-óxido implica la N-oxidación del tramadol. Esto se puede lograr utilizando varios agentes oxidantes como el peróxido de hidrógeno o los perácidos en condiciones controladas . La reacción típicamente requiere un solvente como metanol o etanol y se lleva a cabo a temperatura ambiente para evitar la descomposición del producto.
Métodos de producción industrial: La producción industrial de Tramadol N-óxido sigue rutas sintéticas similares pero a mayor escala. El proceso involucra el uso de reactores de flujo continuo para garantizar la calidad y el rendimiento consistentes del producto. Las condiciones de reacción se optimizan para maximizar la tasa de conversión y minimizar los subproductos .
Análisis De Reacciones Químicas
Tipos de reacciones: El Tramadol N-óxido experimenta varios tipos de reacciones químicas, que incluyen:
Reducción de N-óxido: Esta reacción convierte el Tramadol N-óxido nuevamente a tramadol, que es la forma analgésica activa.
O-desmetilación: Esta reacción implica la eliminación de un grupo metilo del átomo de oxígeno, lo que lleva a la formación de desmetiltramadol.
N-desmetilación: Esta reacción elimina un grupo metilo del átomo de nitrógeno, produciendo nortramadol.
Ciclohexilhidroxilación: Esta reacción agrega un grupo hidroxilo al anillo ciclohexilo.
Reactivos y condiciones comunes:
Reducción de N-óxido: Típicamente involucra el uso de agentes reductores como polvo de zinc o hidrogenación catalítica.
O-desmetilación y N-desmetilación: Estas reacciones a menudo requieren ácidos o bases fuertes, como ácido clorhídrico o hidróxido de sodio, en condiciones de reflujo.
Ciclohexilhidroxilación: Esta reacción generalmente se lleva a cabo utilizando agentes oxidantes como permanganato de potasio u óxido de osmio.
Productos principales:
Tramadol: El producto principal de la reducción de N-óxido.
Desmetiltramadol: El producto de la O-desmetilación.
Nortramadol: El producto de la N-desmetilación.
Derivados de tramadol hidroxilados: Productos de ciclohexilhidroxilación.
Aplicaciones Científicas De Investigación
El Tramadol N-óxido tiene varias aplicaciones de investigación científica, que incluyen:
Biología: Investigado por sus vías metabólicas e interacciones con varias enzimas.
Medicina: Explorado por su potencial como profármaco para mejorar los efectos analgésicos del tramadol.
Industria: Utilizado en el desarrollo de nuevas formulaciones analgésicas y sistemas de administración de fármacos.
Mecanismo De Acción
El Tramadol N-óxido ejerce sus efectos principalmente a través de su conversión a tramadol. El tramadol tiene mecanismos de acción tanto opioides como no opioides. Actúa como un agonista del receptor μ-opioide e inhibe la recaptación de serotonina y norepinefrina . Este doble mecanismo contribuye a sus propiedades analgésicas al modular las señales del dolor en el sistema nervioso central .
Compuestos similares:
Tramadol: El compuesto madre del Tramadol N-óxido, con propiedades analgésicas similares.
Desmetiltramadol: Un metabolito del tramadol con potencia analgésica reducida.
Nortramadol: Otro metabolito con propiedades farmacológicas distintas.
Singularidad: El Tramadol N-óxido es único en su capacidad de servir como un profármaco, proporcionando una liberación sostenida de tramadol y potencialmente reduciendo la frecuencia de dosificación . Su funcionalidad de N-óxido también ofrece una reactividad química distinta, lo que lo convierte en un compuesto valioso para la investigación y el desarrollo en química medicinal .
Comparación Con Compuestos Similares
Tramadol: The parent compound of Tramadol N-oxide, with similar analgesic properties.
Desmethyl tramadol: A metabolite of tramadol with reduced analgesic potency.
Nortramadol: Another metabolite with distinct pharmacological properties.
Uniqueness: this compound is unique in its ability to serve as a prodrug, providing a sustained release of tramadol and potentially reducing the frequency of dosing . Its N-oxide functionality also offers distinct chemical reactivity, making it a valuable compound for research and development in medicinal chemistry .
Actividad Biológica
Tramadol N-Oxide (TNO), also known as RWJ-38705, is a metabolite of tramadol, a widely used analgesic. This compound has gained attention due to its unique pharmacological properties, which differ significantly from those of its parent drug. This article explores the biological activity of TNO, including its metabolism, analgesic effects, and potential clinical applications.
Metabolism of this compound
TNO undergoes extensive metabolism in various species, including humans. Studies have shown that TNO is metabolized primarily in the liver, with significant conversion to tramadol occurring in both rat and human hepatic S9 fractions. The metabolic pathways include:
- N-Oxide Reduction
- O-Demethylation
- N-Demethylation
- Cyclohexylhydroxylation
In vitro studies demonstrated that unchanged TNO constituted 60% of the sample in mouse hepatic fractions, while this percentage was 24% in rats and 26% in humans . The conversion of TNO to tramadol is rapid and substantial, suggesting that TNO acts as a prodrug for tramadol.
Analgesic Activity
TNO exhibits notable analgesic properties that are distinct from those of tramadol. Research indicates that TNO produces dose-related antinociceptive effects across various pain models:
- Mouse Abdominal Irritant Test : ED50 = 15.5 mg/kg
- Hot-Plate Test (48°C) : ED50 = 84.7 mg/kg
- Tail-Flick Test : ED50 = 316.4 mg/kg
- Hot-Plate Test (55°C) : ED50 = 138.2 mg/kg
The duration of action for TNO is significantly extended compared to tramadol, lasting approximately 4-5 hours . Importantly, TNO has minimal affinity for traditional opioid receptors (mu, delta, kappa), indicating a different mechanism of action than typical opioids .
Side Effects and Advantages
TNO offers several advantages over tramadol and traditional opioids:
- Reduced Side Effects : TNO is associated with fewer side effects such as respiratory depression and constipation, which are common with opioid analgesics .
- Longer Duration of Action : Patients may benefit from longer-lasting pain relief without the need for frequent dosing .
- Prodrug Characteristics : As a prodrug, TNO's conversion to tramadol allows for effective pain management while minimizing the risk of abuse associated with direct opioid administration .
Case Studies and Clinical Implications
Case studies have highlighted the potential clinical benefits of TNO:
- Chronic Pain Management : In patients requiring long-term analgesia, TNO may provide effective pain relief with a lower incidence of adverse effects compared to traditional opioids.
- Post-Surgical Pain Control : Studies suggest that TNO could be beneficial in managing postoperative pain due to its prolonged analgesic effects.
Comparative Analysis of Tramadol and this compound
The following table summarizes key differences between tramadol and its N-Oxide derivative:
| Feature | Tramadol | This compound |
|---|---|---|
| Analgesic Mechanism | Opioid receptor agonist | Prodrug; minimal opioid receptor affinity |
| Duration of Action | Shorter (2-4 hours) | Longer (4-5 hours) |
| Side Effects | Respiratory depression, constipation | Fewer side effects |
| Metabolism | Hepatic metabolism | Extensive hepatic metabolism to tramadol |
Propiedades
Fórmula molecular |
C16H25NO3 |
|---|---|
Peso molecular |
279.37 g/mol |
Nombre IUPAC |
1-[(1R,2R)-2-hydroxy-2-(3-methoxyphenyl)cyclohexyl]-N,N-dimethylmethanamine oxide |
InChI |
InChI=1S/C16H25NO3/c1-17(2,19)12-14-7-4-5-10-16(14,18)13-8-6-9-15(11-13)20-3/h6,8-9,11,14,18H,4-5,7,10,12H2,1-3H3/t14-,16+/m1/s1 |
Clave InChI |
HBXKSXMNNGHBEA-ZBFHGGJFSA-N |
SMILES isomérico |
C[N+](C)(C[C@H]1CCCC[C@@]1(C2=CC(=CC=C2)OC)O)[O-] |
SMILES canónico |
C[N+](C)(CC1CCCCC1(C2=CC(=CC=C2)OC)O)[O-] |
Sinónimos |
2-((dimethylamino)methyl)-1-(3-methoxyphenyl)cyclohexanol hydrochloride RWJ 38705 tramadol N-oxide |
Origen del producto |
United States |
Synthesis routes and methods I
Procedure details





Synthesis routes and methods II
Procedure details





Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.













