Floxacrine
Descripción general
Descripción
Floxacrina es un compuesto antimalárico que pertenece a la clase de las fenilquinolinas. Se ha estudiado por su potencial para tratar la malaria, particularmente cepas resistentes a otros medicamentos antimaláricos. Floxacrina y sus derivados han mostrado una actividad prometedora contra varias cepas de Plasmodium, el parásito responsable de la malaria .
Métodos De Preparación
Rutas sintéticas y condiciones de reacción: Floxacrina se puede sintetizar mediante una serie de reacciones químicas que involucran el núcleo de quinolina. Un método común involucra la reacción de acrilato de (N,N)-dimetilamino etilo con aminopropanoles en metilbenceno, seguido de la adición de base de Lewis y trimetilclorosilano para proteger los grupos hidroxilo y amido . La reacción se completa agregando cloruro de tetrafluorobenzoílo, seguido de lavado ácido y eliminación de los grupos protectores .
Métodos de producción industrial: La producción industrial de floxacrina implica optimizar la ruta sintética para aumentar el rendimiento y reducir las impurezas. Esto incluye proteger los grupos hidroxilo y amido usando trimetilclorosilano, lo que mejora la utilización del cloruro de tetrafluorobenzoílo y aumenta el rendimiento de reacción del ácido difluorocarboxílico intermedio en un 10 por ciento .
Análisis De Reacciones Químicas
Tipos de reacciones: Floxacrina se somete a varias reacciones químicas, incluyendo oxidación, reducción y sustitución. Estas reacciones son esenciales para modificar el compuesto para mejorar sus propiedades antimaláricas.
Reactivos y condiciones comunes: Los reactivos comunes utilizados en las reacciones que involucran floxacrina incluyen bases de Lewis, trimetilclorosilano y cloruro de tetrafluorobenzoílo . Las reacciones generalmente se llevan a cabo bajo condiciones controladas para garantizar un alto rendimiento y pureza.
Principales productos formados: Los principales productos formados a partir de las reacciones que involucran floxacrina incluyen varios derivados con actividad antimalárica mejorada. Estos derivados se evalúan por su eficacia contra cepas resistentes a los medicamentos de Plasmodium .
Aplicaciones Científicas De Investigación
Chemical Profile
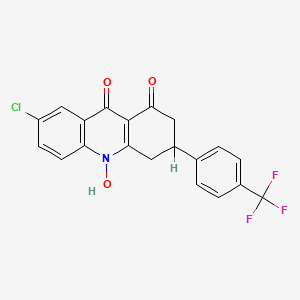
- Chemical Name : 7-chloro-10-hydroxy-3-(4-trifluoromethylphenyl)-3,4-dihydroacridine-1,9(2H, 10H)-dione
- Molecular Formula : C17H15ClF3N
- Molecular Weight : 343.76 g/mol
Antimalarial Activity
Floxacrine has been extensively studied for its efficacy against malaria, particularly in the following areas:
Efficacy Against Plasmodium Species
- Plasmodium berghei :
- Plasmodium cynomolgi :
- Resistance Development :
Comparative Efficacy
This compound's prophylactic effects were found to be superior to primaquine but inferior to pyrimethamine in certain studies . Additionally, it has shown effectiveness against other parasites:
- Eimeria species : Effective at 100 ppm in chickens.
- Fasciola hepatica : Effective at 1000 mg/kg orally in rats.
- Heterakis spumosa : Effective at doses between 300-800 mg/kg orally in rats .
Clinical Trials and Studies
- Study on Blood Schizontocide Activity :
- Prophylactic Studies :
- Comparative Studies with Other Antimalarials :
Summary Table of Efficacy Against Various Parasites
| Parasite | Effective Dose (mg/kg) | Route | Notes |
|---|---|---|---|
| Plasmodium berghei | 0.7 | Oral | Blood schizontocide activity |
| Plasmodium cynomolgi | 0.625 | Oral | Complete prophylaxis |
| Eimeria species | 100 ppm | Oral | Effective in chickens |
| Fasciola hepatica | 1000 | Oral | Effective in rats |
| Heterakis spumosa | 300-800 | Oral | Effective in rats |
Mecanismo De Acción
Floxacrina ejerce sus efectos apuntando a las etapas esquizonticidas sanguíneas de Plasmodium. Interfiere con la capacidad del parásito para replicarse y sobrevivir dentro de los glóbulos rojos del huésped . Los objetivos moleculares exactos y las vías involucradas en su mecanismo de acción aún están bajo investigación, pero se cree que interrumpe los procesos metabólicos del parásito .
Comparación Con Compuestos Similares
Floxacrina se compara con otros compuestos antimaláricos como la cloroquina, la amodiaquina, la isoquinolina y la tebuquina . Si bien estos compuestos han sido efectivos en el tratamiento de la malaria, floxacrina ha mostrado una actividad superior contra cepas resistentes a los medicamentos . Otros compuestos similares incluyen endochin, ICI-56780 y varias quinolonas similares a endochin . La estructura única y el mecanismo de acción de floxacrina la convierten en una adición valiosa al arsenal de medicamentos antimaláricos.
Actividad Biológica
Floxacrine, chemically known as 7-chloro-10-hydroxy-3-(4-trifluoromethylphenyl)-3,4-dihydroacridine-1,9(2H, 10H)-dione, is a compound primarily investigated for its antimalarial properties. It has shown significant efficacy against various strains of malaria parasites, particularly Plasmodium berghei, P. vinckei, and P. cynomolgi. This article synthesizes research findings on the biological activity of this compound, highlighting its mechanisms of action, efficacy in different models, and potential therapeutic applications.
Efficacy Against Malaria Parasites
This compound has demonstrated potent antimalarial activity in both in vitro and in vivo studies:
- Blood Schizontocidal Action : this compound exhibits a high level of activity against both drug-sensitive and drug-resistant strains of Plasmodium berghei. In mouse models, the effective dose (ED50) against sensitive strains was found to be approximately 0.7 mg/kg when administered subcutaneously .
- Resistance Profiles : The compound maintains effectiveness against strains resistant to chloroquine and other common antimalarials. However, resistance can develop with repeated subcurative doses .
Dosage and Administration
This compound's dosage requirements vary significantly based on the infection stage and parasite strain:
| Parasite Strain | Effective Dose (mg/kg) | Administration Route | Comments |
|---|---|---|---|
| P. berghei | 0.7 | Subcutaneous | Effective against sensitive strains |
| P. cynomolgi | 0.625 | Daily during incubation | Complete protection against infection |
| Chloroquine-resistant strains | 1.25 - 2.5 | Daily | Temporary clearance of parasitemia |
The precise mechanism by which this compound exerts its antimalarial effects differs from those of traditional antimalarials like chloroquine. Research indicates that it affects the pigment cytoplasm and nucleus of erythrocytic stages in malaria parasites, leading to structural changes that inhibit their growth .
Study on Owl Monkeys
In a study involving owl monkeys infected with chloroquine-resistant Plasmodium falciparum, this compound was administered at daily doses ranging from 1.25 to 2.5 mg/kg:
- Results : The compound achieved temporary clearance of parasitemia but required significantly higher doses for curing established infections—up to 64 times more than those needed for initial clearance.
- Resistance Development : Rapid development of resistance was noted, indicating the need for careful management in therapeutic settings .
Efficacy in Rodent Models
In rodent studies, this compound was also tested for its prophylactic potential:
- Prophylactic Dosing : A subcutaneous dose showed moderate prophylactic effects due to a "depot effect," where the drug remains active for extended periods .
- Tolerance Levels : The compound demonstrated good tolerance in both rodents and rhesus monkeys, with a wide margin between effective and maximum tolerated doses .
Propiedades
Número CAS |
53966-34-0 |
|---|---|
Fórmula molecular |
C20H13ClF3NO3 |
Peso molecular |
407.8 g/mol |
Nombre IUPAC |
7-chloro-10-hydroxy-3-[4-(trifluoromethyl)phenyl]-3,4-dihydro-2H-acridine-1,9-dione |
InChI |
InChI=1S/C20H13ClF3NO3/c21-13-5-6-15-14(9-13)19(27)18-16(25(15)28)7-11(8-17(18)26)10-1-3-12(4-2-10)20(22,23)24/h1-6,9,11,28H,7-8H2 |
Clave InChI |
AWHZKKVSUJJVNL-UHFFFAOYSA-N |
SMILES |
C1C(CC(=O)C2=C1N(C3=C(C2=O)C=C(C=C3)Cl)O)C4=CC=C(C=C4)C(F)(F)F |
SMILES canónico |
C1C(CC(=O)C2=C1N(C3=C(C2=O)C=C(C=C3)Cl)O)C4=CC=C(C=C4)C(F)(F)F |
Apariencia |
Solid powder |
Pureza |
>98% (or refer to the Certificate of Analysis) |
Vida útil |
>2 years if stored properly |
Solubilidad |
Soluble in DMSO |
Almacenamiento |
Dry, dark and at 0 - 4 C for short term (days to weeks) or -20 C for long term (months to years). |
Sinónimos |
7-chloro-10-hydroxy-3-(4-trifluoromethylphenyl)-3,4-dihydroacridine-1,9(2H,10H)-dione floxacrine HOE 991 |
Origen del producto |
United States |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.
















