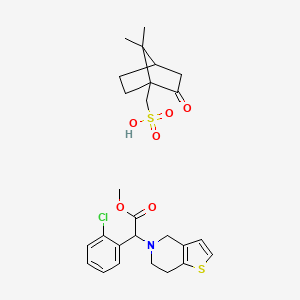
Clopidogrel Camphorsulfonate
- Haga clic en CONSULTA RÁPIDA para recibir una cotización de nuestro equipo de expertos.
- Con productos de calidad a un precio COMPETITIVO, puede centrarse más en su investigación.
Descripción general
Descripción
Clopidogrel Camphorsulfonate is a useful research compound. Its molecular formula is C26H32ClNO6S2 and its molecular weight is 554.1 g/mol. The purity is usually 95%.
BenchChem offers high-quality this compound suitable for many research applications. Different packaging options are available to accommodate customers' requirements. Please inquire for more information about this compound including the price, delivery time, and more detailed information at [email protected].
Aplicaciones Científicas De Investigación
Pharmacological Properties
Clopidogrel is an inhibitor of platelet aggregation, primarily acting by blocking the binding of adenosine diphosphate (ADP) to its receptor on platelets. This mechanism reduces the likelihood of thrombus formation, which is crucial in preventing cardiovascular events such as myocardial infarction and stroke . The camphorsulfonate salt form enhances the stability and solubility of clopidogrel, making it suitable for pharmaceutical formulations .
Key Characteristics
- Stability : Clopidogrel camphorsulfonate exhibits improved stability against moisture and heat compared to other salt forms .
- Solubility : It has satisfactory water solubility, approximately 4.0 mg/ml, which facilitates its dissolution in pharmaceutical compositions .
- Optical Purity : The salt can achieve high optical purity (≥98.5% enantiomeric excess), crucial for ensuring therapeutic efficacy .
Clinical Applications
This compound is primarily indicated for the prevention of atherothrombotic events in various clinical scenarios:
- Acute Coronary Syndrome : It is used in combination with acetylsalicylic acid (ASA) for patients experiencing unstable angina or non-ST-segment elevation myocardial infarction .
- Post-Myocardial Infarction : Administered to prevent further thrombotic events within 35 days following an acute myocardial infarction .
- Ischemic Stroke : Effective in preventing recurrent strokes within six months after an initial event .
- Peripheral Arterial Disease : Used for patients with established peripheral arterial disease to reduce cardiovascular complications .
Efficacy in Post-PCI Patients
A study highlighted that patients undergoing percutaneous coronary intervention (PCI) benefit significantly from clopidogrel therapy, showing a relative risk reduction of 30-85% in major adverse cardiovascular events compared to placebo . This underscores the importance of this compound in high-risk populations.
Pharmacogenetic Considerations
Research indicates that genetic variations affecting the CYP2C19 enzyme can influence the efficacy of clopidogrel. Patients with loss-of-function alleles may experience reduced therapeutic benefits, emphasizing the need for genotype-guided therapy in specific populations .
Comparison with Other Antiplatelet Agents
Clopidogrel has been compared with other antiplatelet medications like ticagrelor and aspirin. Evidence suggests that clopidogrel remains a critical option for patients who may not tolerate newer agents due to side effects or contraindications .
Propiedades
Fórmula molecular |
C26H32ClNO6S2 |
|---|---|
Peso molecular |
554.1 g/mol |
Nombre IUPAC |
(7,7-dimethyl-2-oxo-1-bicyclo[2.2.1]heptanyl)methanesulfonic acid;methyl 2-(2-chlorophenyl)-2-(6,7-dihydro-4H-thieno[3,2-c]pyridin-5-yl)acetate |
InChI |
InChI=1S/C16H16ClNO2S.C10H16O4S/c1-20-16(19)15(12-4-2-3-5-13(12)17)18-8-6-14-11(10-18)7-9-21-14;1-9(2)7-3-4-10(9,8(11)5-7)6-15(12,13)14/h2-5,7,9,15H,6,8,10H2,1H3;7H,3-6H2,1-2H3,(H,12,13,14) |
Clave InChI |
XEENARPWPCQXST-UHFFFAOYSA-N |
SMILES canónico |
CC1(C2CCC1(C(=O)C2)CS(=O)(=O)O)C.COC(=O)C(C1=CC=CC=C1Cl)N2CCC3=C(C2)C=CS3 |
Origen del producto |
United States |
Synthesis routes and methods
Procedure details





Descargo de responsabilidad e información sobre productos de investigación in vitro
Tenga en cuenta que todos los artículos e información de productos presentados en BenchChem están destinados únicamente con fines informativos. Los productos disponibles para la compra en BenchChem están diseñados específicamente para estudios in vitro, que se realizan fuera de organismos vivos. Los estudios in vitro, derivados del término latino "in vidrio", involucran experimentos realizados en entornos de laboratorio controlados utilizando células o tejidos. Es importante tener en cuenta que estos productos no se clasifican como medicamentos y no han recibido la aprobación de la FDA para la prevención, tratamiento o cura de ninguna condición médica, dolencia o enfermedad. Debemos enfatizar que cualquier forma de introducción corporal de estos productos en humanos o animales está estrictamente prohibida por ley. Es esencial adherirse a estas pautas para garantizar el cumplimiento de los estándares legales y éticos en la investigación y experimentación.














