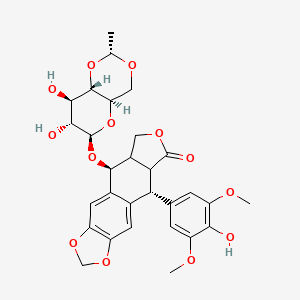
Etoposide
Vue d'ensemble
Description
L’étoposide est un agent chimiothérapeutique utilisé principalement dans le traitement de divers types de cancer, notamment le cancer des testicules, le cancer du poumon, le lymphome, la leucémie, le neuroblastome et le cancer de l’ovaire . Il s’agit d’un dérivé semisynthétique de la podophyllotoxine, un produit naturel extrait des racines et des rhizomes des plantes du genre Podophyllum . L’étoposide agit en inhibant l’enzyme topoisomérase II, essentielle à la réplication de l’ADN et à la division cellulaire .
Méthodes De Préparation
L’étoposide est synthétisé par un processus semisynthétique à partir de la podophyllotoxine. Une méthode courante implique la condensation directe de la 4’-déméthylepipodophyllotoxine avec le 2,3-di-O-dichloroacétyl-(4,6-O-éthylidène)-β-D-glucopyranose en présence de triméthylsilyl trifluorométhanesulfonate (TMSOTf) pour obtenir la 4’-déméthylepipodophyllotoxine-4-(2,3-di-O-dichloroacétyl-4,6-0-éthylidène)-β-D-glucopyranoside, qui est ensuite converti en étoposide . Cette méthode offre des rendements améliorés, des temps de réaction réduits et des procédures d’isolement plus favorables par rapport aux techniques existantes .
Analyse Des Réactions Chimiques
L’étoposide subit plusieurs types de réactions chimiques, notamment :
Réduction : Les réactions de réduction peuvent convertir l’étoposide en sa forme hydroquinone.
Substitution : L’étoposide peut subir des réactions de substitution, en particulier au niveau du fragment glucopyranoside.
Les réactifs et les conditions couramment utilisés dans ces réactions comprennent des oxydants comme le peroxyde d’hydrogène et des réducteurs comme le borohydrure de sodium. Les principaux produits formés à partir de ces réactions comprennent les dérivés O-quinone et hydroquinone de l’étoposide .
Applications de la recherche scientifique
L’étoposide a un large éventail d’applications de recherche scientifique, notamment :
Applications De Recherche Scientifique
Etoposide is a semi-synthetic derivative of podophyllotoxin that inhibits topoisomerase-II, leading to DNA strand breaks and apoptosis . Since its FDA approval in 1983, this compound has been used to treat solid and hematologic tumors, including lung cancer, germ cell tumors, and lymphoma .
This compound in Other Cancer Treatments
This compound, often in combination with other chemotherapeutic agents, is used to treat various cancers .
- Non-Hodgkin's Lymphoma this compound, combined with lomustine, methotrexate, and prednisone, is a first-line therapy for non-Hodgkin's lymphoma without major cardiotoxicity .
- Hodgkin's Disease this compound is a first-line chemotherapeutic agent combined with vincristine, chloramphenicol, and prednisolone, with a 77% response rate .
- Brain Tumors this compound has been observed to work against brain tumors in combination therapy with cyclophosphamide, cisplatin, vincristine, and carboplatin .
- Breast Cancer this compound has been explored in the management of metastatic breast cancer . A single-agent trial in untreated patients showed a response rate of approximately 15% .
- Germ Cell Tumors Adjuvant this compound plus cisplatin for 2 cycles has demonstrated prolonged disease-specific and relapse-free survival with acceptable toxicity and lower drug costs in patients with nonseminomatous germ cell tumors .
- Choriocarcinoma Low-dose this compound and cisplatin (EP) can be effective in treating elderly patients with choriocarcinoma .
Adverse Events and Toxicity
Mécanisme D'action
L’étoposide exerce ses effets en inhibant l’enzyme topoisomérase II, qui est responsable de la régulation de la topologie de l’ADN par un mécanisme de passage à double brin . En formant un complexe avec la topoisomérase II et l’ADN, l’étoposide induit des ruptures dans l’ADN double brin et empêche la réparation par la liaison de la topoisomérase II . L’accumulation de ruptures dans l’ADN empêche l’entrée dans la phase mitotique de la division cellulaire, conduisant à la mort cellulaire . L’étoposide agit principalement dans les phases G2 et S du cycle cellulaire .
Comparaison Avec Des Composés Similaires
L’étoposide est similaire à d’autres dérivés de la podophyllotoxine, tels que la téniposide et la podophyllotoxine elle-même. L’étoposide est unique en raison de son inhibition spécifique de la topoisomérase II et de son utilisation dans un large éventail de traitements anticancéreux .
Podophyllotoxine : Le produit naturel dont l’étoposide est dérivé, utilisé principalement pour ses propriétés antivirales et antimitotiques.
La singularité de l’étoposide réside dans sa nature semisynthétique, sa stabilité accrue et son large spectre d’activité anticancéreuse .
Activité Biologique
Etoposide is a semisynthetic derivative of podophyllotoxin, primarily utilized as an antineoplastic agent in cancer therapy. Its biological activity is predominantly characterized by its ability to inhibit DNA topoisomerase II, leading to significant effects on DNA synthesis and cell cycle progression. This article delves into the mechanisms of action, clinical applications, and recent research findings regarding this compound's biological activity.
This compound exerts its effects primarily through the following mechanisms:
- Inhibition of DNA Topoisomerase II : this compound forms a complex with topoisomerase II, preventing the re-ligation of DNA strands after they have been cleaved. This results in the accumulation of DNA double-strand breaks (DSBs), which can trigger apoptosis in cancer cells .
- Cell Cycle Specificity : The drug is cell cycle-dependent, affecting mainly the S and G2 phases. At high concentrations, it can induce cell lysis during mitosis, while lower concentrations inhibit cells from entering prophase .
- Activation of DNA Damage Response : this compound activates key proteins involved in the DNA damage response, such as ATM (Ataxia Telangiectasia Mutated) kinase, which leads to the formation of repair foci and chromosomal abnormalities if DSBs are not repaired .
Clinical Applications
This compound is widely used in various chemotherapy regimens for different types of cancers, particularly small-cell lung cancer (SCLC) and testicular cancer. Its effectiveness can be influenced by the scheduling of administration:
- Small-Cell Lung Cancer : In a randomized trial comparing two administration schedules, the five-day infusion regimen demonstrated an overall response rate of 89%, significantly higher than the 10% observed with a 24-hour continuous infusion schedule .
- Combination Therapies : this compound is often combined with other agents. For instance, in treating extensive SCLC, combinations with cisplatin have shown objective response rates ranging from 44% to 78% .
Research Findings and Case Studies
Recent studies have expanded our understanding of this compound's biological activity beyond its traditional uses:
- Antibacterial Activity : A study explored this compound's antibacterial properties when combined with hydroxyapatite, suggesting potential repurposing for treating infections .
- Pediatric Oncology : The OLIE trial evaluated the combination of lenvatinib, ifosfamide, and this compound in children with high-grade osteosarcoma, showing promising outcomes .
- Toxicity Management : Research has indicated that this compound can cause myelosuppressive toxicity; however, recent trials have focused on optimizing dosing regimens to mitigate these effects while maintaining efficacy .
Data Summary
The following table summarizes key findings from various studies on this compound:
Q & A
Basic Research Questions
Q. What is the primary mechanism by which etoposide exerts its anticancer effects?
this compound targets DNA topoisomerase II (Topo II), stabilizing the enzyme-DNA cleavage complex and preventing religation of DNA strands. This results in double-strand breaks (DSBs) and triggers apoptosis via activation of stress-associated signaling pathways (e.g., p53-mediated cell cycle arrest) . Notably, only 0.3% of this compound-induced strand breaks activate H2AX phosphorylation, suggesting most damage is non-toxic .
Q. How can researchers design experiments to assess this compound-induced cytotoxicity in vitro?
Standard protocols include measuring lactate dehydrogenase (LDH) release as a cell death marker, flow cytometry for cell cycle distribution (e.g., G2/M arrest), and apoptosis assays (Annexin V/PI staining). For example, SIN-1-treated astrocytes under glucose deprivation showed this compound's cytoprotective effect via LDH release quantification .
Q. What factors influence the variable oral bioavailability of this compound, and how can these be addressed preclinically?
this compound's low solubility (BCS Class IV) and rapid clearance (terminal half-life: 1.5 hours) limit bioavailability (24–74%). Researchers use nanosuspensions stabilized with surfactants (e.g., PLGA nanoparticles) and optimize formulations via Box–Behnken factorial designs to enhance dissolution and sustained release .
Advanced Research Questions
Q. How can experimental design mitigate biases in studies comparing this compound combination therapies (e.g., cisplatin + this compound)?
Rigorous protocols involve blinding, randomization, and standardized dosing. A meta-analysis of small-cell lung cancer trials highlighted the importance of controlling for confounders like patient stratification and toxicity metrics (e.g., myelosuppression rates) to validate efficacy claims .
Q. What molecular mechanisms underlie this compound resistance, and how can they be systematically investigated?
Resistance arises via reduced Topo II expression, overexpression of drug efflux pumps (ABCC1/ABCC6), or dysregulated signaling pathways (JAK-STAT, MAPK). Gene expression profiling (microarrays, RNA-seq) and functional assays (e.g., siRNA knockdown) in resistant cell lines (e.g., MCF7VP) reveal key pathways . Fluctuation analysis in MES-SA cells estimated mutation rates (2.9 × 10⁻⁶ to 1.7 × 10⁻⁷ per generation) for spontaneous resistance .
Q. How do contradictory findings on this compound's dual role in cytotoxicity and cytoprotection arise, and how can they be resolved?
this compound scavenges peroxynitrite (ONOO⁻) in glucose-deprived astrocytes, reducing oxidative stress independent of cell cycle inhibition . Contradictions may stem from model-specific variables (e.g., cell type, stress conditions). Researchers should validate mechanisms using multiple assays (e.g., glutathione depletion, superoxide anion measurement) .
Q. What advanced formulations improve this compound's pharmacokinetics, and how are they optimized?
Amorphous nanopowders (particle size: ~200 nm) using freeze-drying with cryoprotectants (mannitol) enhance oral absorption (Cmax: 2.21×; AUC: 2.13× vs. coarse powder). Optimization involves response surface methodology for ultrasonication time, phase ratios, and stabilizer concentration .
Q. Methodological Guidance
Q. How should researchers statistically analyze survival data from this compound-treated cohorts?
Use Kaplan-Meier survival curves with log-rank tests for significance. A meta-analysis of high-grade glioma trials reported median survival gains (SG: 7–23 months) and stratified results by treatment regimen (e.g., VP-16 vs. VM-26) .
Q. What in vitro models best recapitulate this compound's blood-brain barrier penetration for CNS cancer studies?
Primary rat astrocyte cultures under glucose deprivation and oxidative stress (SIN-1 exposure) mimic the blood-brain barrier's metabolic constraints. Measure NO release with Clark-type electrodes and superoxide with lucigenin chemiluminescence .
Q. How can researchers validate this compound's target engagement in DNA damage assays?
Comet assays quantify DSBs/SSBs, while γ-H2AX immunofluorescence marks repair foci. Calicheamicin-induced DSBs serve as positive controls to distinguish Topo II-dependent damage .
Propriétés
Numéro CAS |
33419-42-0 |
|---|---|
Formule moléculaire |
C29H32O13 |
Poids moléculaire |
588.6 g/mol |
Nom IUPAC |
(5S,5aR,8aR,9R)-5-[[(2R,4aR,6R,7S,8R,8aS)-7,8-dihydroxy-2-methyl-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxin-6-yl]oxy]-9-(4-hydroxy-3,5-dimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-[2]benzofuro[6,5-f][1,3]benzodioxol-8-one |
InChI |
InChI=1S/C29H32O13/c1-11-36-9-20-27(40-11)24(31)25(32)29(41-20)42-26-14-7-17-16(38-10-39-17)6-13(14)21(22-15(26)8-37-28(22)33)12-4-18(34-2)23(30)19(5-12)35-3/h4-7,11,15,20-22,24-27,29-32H,8-10H2,1-3H3/t11-,15+,20-,21-,22+,24-,25+,26-,27-,29+/m1/s1 |
Clé InChI |
VJJPUSNTGOMMGY-QBUITQBFSA-N |
Impuretés |
The following impurities are limited by the requirements of The British Pharmacopoeia: 4'-carbenzoxy ethylidene lignan P, picroethylidene lignan P, alpha-ethylidene lignan P, lignan P and 4'-demethylepipodophyllotoxin. |
SMILES |
CC1OCC2C(O1)C(C(C(O2)OC3C4COC(=O)C4C(C5=CC6=C(C=C35)OCO6)C7=CC(=C(C(=C7)OC)O)OC)O)O |
SMILES isomérique |
C[C@@H]1OC[C@@H]2[C@@H](O1)[C@@H]([C@@H]([C@@H](O2)O[C@H]3[C@H]4COC(=O)[C@@H]4[C@@H](C5=CC6=C(C=C35)OCO6)C7=CC(=C(C(=C7)OC)O)OC)O)O |
SMILES canonique |
CC1OCC2C(O1)C(C(C(O2)OC3C4COC(=O)C4C(C5=CC6=C(C=C35)OCO6)C7=CC(=C(C(=C7)OC)O)OC)O)O |
Apparence |
White to off-white solid powder |
Color/Form |
Crystals from methanol |
melting_point |
236-251 °C |
Key on ui other cas no. |
33419-42-0 |
Description physique |
Solid |
Pictogrammes |
Irritant; Health Hazard |
Pureté |
>98% (or refer to the Certificate of Analysis) |
Durée de conservation |
>2 years if stored properly |
Solubilité |
Very soluble in methanol, chloroform; slightly soluble in ethanol, sparingly soluble in water. Sol in alc: approx 0.76 mg/ml Water solubility: approx 0.08 mg/mL |
Stockage |
Dry, dark and at 0 - 4 C for short term (days to weeks) or -20 C for long term (months to years). |
Synonymes |
alpha-D-Glucopyranosyl Isomer Etoposide Celltop Demethyl Epipodophyllotoxin Ethylidine Glucoside Eposide Eposin Eto GRY Eto-GRY Etomedac Etopos Etoposide Etoposide Pierre Fabre Etoposide Teva Etoposide, (5a alpha)-Isomer Etoposide, (5a alpha,9 alpha)-Isomer Etoposide, (5S)-Isomer Etoposide, alpha D Glucopyranosyl Isomer Etoposide, alpha-D-Glucopyranosyl Isomer Etoposido Ferrer Farma Exitop Lastet NSC 141540 NSC-141540 NSC141540 Onkoposid Riboposid Teva, Etoposide Toposar Vépéside Sandoz Vépéside-Sandoz Vepesid VP 16 VP 16 213 VP 16-213 VP 16213 VP-16 VP16 |
Pression de vapeur |
5.4X10-23 mm Hg at 25 °C /Estimated/ |
Origine du produit |
United States |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Avertissement et informations sur les produits de recherche in vitro
Veuillez noter que tous les articles et informations sur les produits présentés sur BenchChem sont destinés uniquement à des fins informatives. Les produits disponibles à l'achat sur BenchChem sont spécifiquement conçus pour des études in vitro, qui sont réalisées en dehors des organismes vivants. Les études in vitro, dérivées du terme latin "in verre", impliquent des expériences réalisées dans des environnements de laboratoire contrôlés à l'aide de cellules ou de tissus. Il est important de noter que ces produits ne sont pas classés comme médicaments et n'ont pas reçu l'approbation de la FDA pour la prévention, le traitement ou la guérison de toute condition médicale, affection ou maladie. Nous devons souligner que toute forme d'introduction corporelle de ces produits chez les humains ou les animaux est strictement interdite par la loi. Il est essentiel de respecter ces directives pour assurer la conformité aux normes légales et éthiques en matière de recherche et d'expérimentation.
















