Floxacrine
Overview
Description
Floxacrine is an antimalarial compound belonging to the class of phenylquinolines. It has been studied for its potential to treat malaria, particularly strains resistant to other antimalarial drugs. This compound and its derivatives have shown promising activity against various strains of Plasmodium, the parasite responsible for malaria .
Preparation Methods
Synthetic Routes and Reaction Conditions: Floxacrine can be synthesized through a series of chemical reactions involving the quinoline core. One common method involves the reaction of (N,N)-dimethylamino ethyl acrylate with aminopropanols in methylbenzene, followed by the addition of lewis base and trimethylchlorosilane to protect hydroxyl and amido groups . The reaction is completed by adding tetrafluorobenzoyl chloride, followed by acid washing and removal of protecting groups .
Industrial Production Methods: Industrial production of this compound involves optimizing the synthetic route to increase yield and reduce impurities. This includes protecting hydroxyl and amido groups using trimethylchlorosilane, which enhances the utilization of tetrafluorobenzoyl chloride and increases the reaction yield of intermediate difluorocarboxylic acid by 10 percent .
Chemical Reactions Analysis
Types of Reactions: Floxacrine undergoes various chemical reactions, including oxidation, reduction, and substitution. These reactions are essential for modifying the compound to enhance its antimalarial properties.
Common Reagents and Conditions: Common reagents used in the reactions involving this compound include lewis bases, trimethylchlorosilane, and tetrafluorobenzoyl chloride . The reactions are typically carried out under controlled conditions to ensure high yield and purity.
Major Products Formed: The major products formed from the reactions involving this compound include various derivatives with enhanced antimalarial activity. These derivatives are evaluated for their efficacy against drug-resistant strains of Plasmodium .
Scientific Research Applications
Chemical Profile
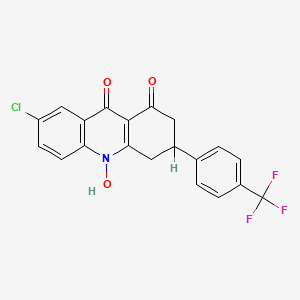
- Chemical Name : 7-chloro-10-hydroxy-3-(4-trifluoromethylphenyl)-3,4-dihydroacridine-1,9(2H, 10H)-dione
- Molecular Formula : C17H15ClF3N
- Molecular Weight : 343.76 g/mol
Antimalarial Activity
Floxacrine has been extensively studied for its efficacy against malaria, particularly in the following areas:
Efficacy Against Plasmodium Species
- Plasmodium berghei :
- Plasmodium cynomolgi :
- Resistance Development :
Comparative Efficacy
This compound's prophylactic effects were found to be superior to primaquine but inferior to pyrimethamine in certain studies . Additionally, it has shown effectiveness against other parasites:
- Eimeria species : Effective at 100 ppm in chickens.
- Fasciola hepatica : Effective at 1000 mg/kg orally in rats.
- Heterakis spumosa : Effective at doses between 300-800 mg/kg orally in rats .
Clinical Trials and Studies
- Study on Blood Schizontocide Activity :
- Prophylactic Studies :
- Comparative Studies with Other Antimalarials :
Summary Table of Efficacy Against Various Parasites
| Parasite | Effective Dose (mg/kg) | Route | Notes |
|---|---|---|---|
| Plasmodium berghei | 0.7 | Oral | Blood schizontocide activity |
| Plasmodium cynomolgi | 0.625 | Oral | Complete prophylaxis |
| Eimeria species | 100 ppm | Oral | Effective in chickens |
| Fasciola hepatica | 1000 | Oral | Effective in rats |
| Heterakis spumosa | 300-800 | Oral | Effective in rats |
Mechanism of Action
Floxacrine exerts its effects by targeting the blood schizontocidal stages of Plasmodium. It interferes with the parasite’s ability to replicate and survive within the host’s red blood cells . The exact molecular targets and pathways involved in its mechanism of action are still under investigation, but it is believed to disrupt the parasite’s metabolic processes .
Comparison with Similar Compounds
Floxacrine is compared with other antimalarial compounds such as chloroquine, amodiaquine, isoquine, and tebuquine . While these compounds have been effective in treating malaria, this compound has shown superior activity against drug-resistant strains . Other similar compounds include endochin, ICI-56780, and various endochin-like quinolones . This compound’s unique structure and mechanism of action make it a valuable addition to the arsenal of antimalarial drugs.
Biological Activity
Floxacrine, chemically known as 7-chloro-10-hydroxy-3-(4-trifluoromethylphenyl)-3,4-dihydroacridine-1,9(2H, 10H)-dione, is a compound primarily investigated for its antimalarial properties. It has shown significant efficacy against various strains of malaria parasites, particularly Plasmodium berghei, P. vinckei, and P. cynomolgi. This article synthesizes research findings on the biological activity of this compound, highlighting its mechanisms of action, efficacy in different models, and potential therapeutic applications.
Efficacy Against Malaria Parasites
This compound has demonstrated potent antimalarial activity in both in vitro and in vivo studies:
- Blood Schizontocidal Action : this compound exhibits a high level of activity against both drug-sensitive and drug-resistant strains of Plasmodium berghei. In mouse models, the effective dose (ED50) against sensitive strains was found to be approximately 0.7 mg/kg when administered subcutaneously .
- Resistance Profiles : The compound maintains effectiveness against strains resistant to chloroquine and other common antimalarials. However, resistance can develop with repeated subcurative doses .
Dosage and Administration
This compound's dosage requirements vary significantly based on the infection stage and parasite strain:
| Parasite Strain | Effective Dose (mg/kg) | Administration Route | Comments |
|---|---|---|---|
| P. berghei | 0.7 | Subcutaneous | Effective against sensitive strains |
| P. cynomolgi | 0.625 | Daily during incubation | Complete protection against infection |
| Chloroquine-resistant strains | 1.25 - 2.5 | Daily | Temporary clearance of parasitemia |
The precise mechanism by which this compound exerts its antimalarial effects differs from those of traditional antimalarials like chloroquine. Research indicates that it affects the pigment cytoplasm and nucleus of erythrocytic stages in malaria parasites, leading to structural changes that inhibit their growth .
Study on Owl Monkeys
In a study involving owl monkeys infected with chloroquine-resistant Plasmodium falciparum, this compound was administered at daily doses ranging from 1.25 to 2.5 mg/kg:
- Results : The compound achieved temporary clearance of parasitemia but required significantly higher doses for curing established infections—up to 64 times more than those needed for initial clearance.
- Resistance Development : Rapid development of resistance was noted, indicating the need for careful management in therapeutic settings .
Efficacy in Rodent Models
In rodent studies, this compound was also tested for its prophylactic potential:
- Prophylactic Dosing : A subcutaneous dose showed moderate prophylactic effects due to a "depot effect," where the drug remains active for extended periods .
- Tolerance Levels : The compound demonstrated good tolerance in both rodents and rhesus monkeys, with a wide margin between effective and maximum tolerated doses .
Q & A
Basic Research Question: What experimental models are most appropriate for assessing Floxacrine's antimalarial efficacy in preclinical studies?
Methodological Answer:
To evaluate this compound’s efficacy, researchers should employ a tiered approach:
- In vitro assays : Use standardized Plasmodium falciparum culture systems (e.g., SYBR Green assays) to measure IC₅₀ values. Ensure consistency by adhering to WHO protocols for antimalarial drug testing .
- In vivo models : Utilize murine malaria models (e.g., P. berghei-infected mice) to assess parasitemia reduction and survival rates. Optimize dosing regimens based on pharmacokinetic data, ensuring compliance with ethical guidelines for animal studies .
- Controls : Include reference drugs (e.g., chloroquine) and vehicle controls to validate assay sensitivity .
Advanced Research Question: How can researchers resolve contradictions in reported data on this compound’s mechanism of action across studies?
Methodological Answer:
Addressing contradictions requires:
- Systematic review : Conduct a meta-analysis of existing studies, prioritizing those with transparent methodologies (e.g., clear descriptions of parasite strains, assay conditions) .
- Mechanistic redundancy checks : Use orthogonal techniques (e.g., CRISPR-Cas9 gene editing to validate target engagement vs. biochemical assays) to confirm hypotheses .
- Data triangulation : Compare findings across models (e.g., in vitro vs. ex vivo erythrocyte models) to identify context-dependent effects .
Basic Research Question: What are the key parameters for optimizing this compound’s solubility and stability in formulation studies?
Methodological Answer:
- Solubility enhancement : Test co-solvents (e.g., PEG 400) and surfactants (e.g., Tween 80) using phase solubility diagrams. Validate via HPLC under varying pH conditions .
- Stability profiling : Conduct accelerated stability studies (40°C/75% RH for 6 months) and monitor degradation products via LC-MS .
- Bioavailability correlation : Cross-reference solubility data with in vivo pharmacokinetic profiles to ensure translational relevance .
Advanced Research Question: How can researchers design studies to investigate this compound’s potential resistance mechanisms in malaria parasites?
Methodological Answer:
- Resistance induction : Expose P. falciparum cultures to sub-therapeutic this compound doses over multiple generations. Sequence resistant strains to identify mutations (e.g., pfcrt or pfmdr1 polymorphisms) .
- Functional validation : Use allelic replacement assays to confirm causal mutations .
- Cross-resistance screening : Test resistant strains against structurally related antimalarials (e.g., pyronaridine) to map resistance pathways .
Basic Research Question: What statistical methods are recommended for analyzing dose-response relationships in this compound studies?
Methodological Answer:
- Non-linear regression : Fit dose-response curves using a four-parameter logistic model (e.g., GraphPad Prism) to calculate EC₅₀/EC₉₀ values .
- Outlier management : Apply Grubbs’ test to exclude anomalous data points while maintaining biological relevance .
- Replicability checks : Report intra- and inter-assay variability metrics (e.g., coefficient of variation <15%) .
Advanced Research Question: How can synergistic effects between this compound and partner drugs be rigorously quantified?
Methodological Answer:
- Isobologram analysis : Plot combinations at fixed ratios and calculate combination indices (CI <1 indicates synergy) .
- Mechanistic complementarity : Pair this compound (a mitochondrial electron transport inhibitor) with drugs targeting unrelated pathways (e.g., artemisinins acting on heme detoxification) .
- Time-kill assays : Monitor parasite viability over 72 hours to distinguish additive vs. synergistic effects .
Basic Research Question: What ethical considerations are critical when designing clinical trials for this compound?
Methodological Answer:
- Informed consent : Ensure participants in malaria-endemic regions receive culturally adapted explanations of risks/benefits .
- Data safety monitoring : Establish independent boards to review adverse events, particularly in pediatric or pregnant populations .
- Equitable access : Outline post-trial access plans for this compound in resource-limited settings .
Advanced Research Question: How can multi-omics approaches elucidate this compound’s off-target effects in host cells?
Methodological Answer:
- Transcriptomics : Perform RNA-seq on this compound-treated hepatocytes to identify dysregulated pathways (e.g., oxidative stress responses) .
- Proteomics : Use SILAC labeling to quantify changes in host protein expression post-treatment .
- Integration tools : Apply systems biology platforms (e.g., Cytoscape) to map cross-omics interactions and prioritize validation targets .
Guidelines for Further Research:
Properties
CAS No. |
53966-34-0 |
|---|---|
Molecular Formula |
C20H13ClF3NO3 |
Molecular Weight |
407.8 g/mol |
IUPAC Name |
7-chloro-10-hydroxy-3-[4-(trifluoromethyl)phenyl]-3,4-dihydro-2H-acridine-1,9-dione |
InChI |
InChI=1S/C20H13ClF3NO3/c21-13-5-6-15-14(9-13)19(27)18-16(25(15)28)7-11(8-17(18)26)10-1-3-12(4-2-10)20(22,23)24/h1-6,9,11,28H,7-8H2 |
InChI Key |
AWHZKKVSUJJVNL-UHFFFAOYSA-N |
SMILES |
C1C(CC(=O)C2=C1N(C3=C(C2=O)C=C(C=C3)Cl)O)C4=CC=C(C=C4)C(F)(F)F |
Canonical SMILES |
C1C(CC(=O)C2=C1N(C3=C(C2=O)C=C(C=C3)Cl)O)C4=CC=C(C=C4)C(F)(F)F |
Appearance |
Solid powder |
Purity |
>98% (or refer to the Certificate of Analysis) |
shelf_life |
>2 years if stored properly |
solubility |
Soluble in DMSO |
storage |
Dry, dark and at 0 - 4 C for short term (days to weeks) or -20 C for long term (months to years). |
Synonyms |
7-chloro-10-hydroxy-3-(4-trifluoromethylphenyl)-3,4-dihydroacridine-1,9(2H,10H)-dione floxacrine HOE 991 |
Origin of Product |
United States |
Retrosynthesis Analysis
AI-Powered Synthesis Planning: Our tool employs the Template_relevance Pistachio, Template_relevance Bkms_metabolic, Template_relevance Pistachio_ringbreaker, Template_relevance Reaxys, Template_relevance Reaxys_biocatalysis model, leveraging a vast database of chemical reactions to predict feasible synthetic routes.
One-Step Synthesis Focus: Specifically designed for one-step synthesis, it provides concise and direct routes for your target compounds, streamlining the synthesis process.
Accurate Predictions: Utilizing the extensive PISTACHIO, BKMS_METABOLIC, PISTACHIO_RINGBREAKER, REAXYS, REAXYS_BIOCATALYSIS database, our tool offers high-accuracy predictions, reflecting the latest in chemical research and data.
Strategy Settings
| Precursor scoring | Relevance Heuristic |
|---|---|
| Min. plausibility | 0.01 |
| Model | Template_relevance |
| Template Set | Pistachio/Bkms_metabolic/Pistachio_ringbreaker/Reaxys/Reaxys_biocatalysis |
| Top-N result to add to graph | 6 |
Feasible Synthetic Routes
Disclaimer and Information on In-Vitro Research Products
Please be aware that all articles and product information presented on BenchChem are intended solely for informational purposes. The products available for purchase on BenchChem are specifically designed for in-vitro studies, which are conducted outside of living organisms. In-vitro studies, derived from the Latin term "in glass," involve experiments performed in controlled laboratory settings using cells or tissues. It is important to note that these products are not categorized as medicines or drugs, and they have not received approval from the FDA for the prevention, treatment, or cure of any medical condition, ailment, or disease. We must emphasize that any form of bodily introduction of these products into humans or animals is strictly prohibited by law. It is essential to adhere to these guidelines to ensure compliance with legal and ethical standards in research and experimentation.